1797
Multi-voxel PRESS for fast and sensitive static MRS of hyperpolarized nuclei1Department of Nuclear Medicine, TUM School of Medicine, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany
Synopsis
A multi-voxel point resolved spectroscopy (MV-PRESS) sequence was developed for measuring hyperpolarized 13C-labelled compounds, on a 7T preclinical small animal scanner. MV-PRESS allows to excite selected imaging voxels within a 3D volume with high sensitivity (i.e. 90° excitation angle). This method was compared to both single-voxel PRESS of a thermal 1H phantom and CSI with a hyperpolarized [1-13C]pyruvate phantom. MV-PRESS was then used to detect both pyruvate and lactate in healthy mouse kidneys after hyperpolarized [1-13C]pyruvate injection, using 2x2x2 mm3 voxels for a total of 4 voxels.
Introduction
Hyperpolarization can increase the NMR signal by a factor of more than 10.0001, but this enhancement is rapidly consumed due to dilution of the hyperpolarized agents after injection, rapid T1 relaxation and pulsed excitations. As a result, the detection of hyperpolarized signals is still limited by their signal-to-noise ratio (SNR). Several fast and efficient pulse sequences for spectroscopy and imaging of hyperpolarized compounds have been developed trading SNR between spatial, temporal and spectral resolution2. Specifically for static acquisitions, single-voxel PRESS has been suggested as a method to achieve high spectral resolution together with high SNR. In this work, we introduce a multi-voxel point resolved spectroscopy sequence for high sensitivity localized MRS of hyperpolarized nuclei.Methods
MR System: Small animal 7T preclinical scanner (Agilent/GE magnet, Bruker AVANCE III HD electronics) using a dual-tuned 1H/13C volume resonator (inner diameter 31 mm, RAPID Biomedical).Hyperpolarization: 22-28 mg 14M [1-13C]pyruvate was polarized to 23±9% in a HyperSense DNP (Oxford Instruments) at 1.2 K, and then rapidly dissolved in buffer saline.
Phantoms: Thermal 1H: Three 5mm NMR glass tubes filled with water, 80% ethanol and canola oil. Hyperpolarized (HP) 13C: 2ml plastic Eppendorf tube filled with the solution stated above.
Animal Subject: One healthy female C57BL/6 mouse (21.6 g), anesthetized with isoflurane and injected with 300 µl of HP [1-13C]pyruvate via a tail vein catheter.
Proton Imaging: For phantom measurements, T1-weighted FLASH images were acquired as anatomical reference for positioning voxels. For in vivo anatomical reference, T2-weighted RARE images were acquired.
Spectroscopy: 1H spectra for both the single-voxel (SV) and multi-voxel PRESS (MV) sequence were measured (TR= 2000 ms, TE=20 ms, voxel size=1x1x1 mm3, receiver bandwidth=20 ppm, spectral acquisition points=1024).
For hyperpolarized phantom measurements, 13C transmit power was calibrated using a thermal [1-13C]lactate phantom. Reference imaging for voxel positioning was done on a same-size water-filled Eppendorf tube placed at the same position as the later hyperpolarized phantom. A potential spatial overlap of excitation and refocusing slices for all MRS voxels was excluded by a software tool written in Matlab® (The MathWorks Inc.).
Spectra of hyperpolarized [1-13C]pyruvate were acquired using a slice selective 2D FID-CSI sequence (TR=100 ms, TE=1.2 ms, flip angle=12°, matrix size=12x12, voxel size=1x1x1 mm3, spectral acquisition points=256, starting 47 s after start of polarizer dissolution). FID-CSI was followed directly by the MV-PRESS sequence (TR=500 ms, TE=15 ms, 4 voxels, voxel size=1x1x1 mm3, receiver bandwidth=40 ppm, spectral acquisition points=1024).
In vivo 13C spectra were measured using the MV-PRESS sequence (TR=2000 ms, TE=20 ms, 4 voxels, voxel size=2x2x2 mm3, receiver bandwidth=60 ppm, spectral acquisition points=2048), starting approximately 30 seconds after the dissolution and 10 seconds after the end of the injection.
Data Analysis: All data was analyzed in Python 3.83,4. Line broadening (10 Hz) was applied to PRESS spectra for the hyperpolarized in vivo measurement.
Results
Initial 1H measurements show that the MV-PRESS sequence excites and reads out the same voxels as a stock single voxel PRESS sequence (Fig. 1). Spectra for water, oil and ethanol appear close to identical for both sequences.Fig. 2 depicts the results of the hyperpolarized [1-13C]pyruvate phantom measurement. Fig. 2A is the spectrum acquired from one of the four voxels marked in the coronal slice (Fig. 2C) that is closest to the CSI-slice. Fig. 2B shows the spectrum from a voxel of the CSI centered in axial orientation through the tube. A SNR analysis of both spectra is difficult because of ringing artefacts in the CSI that are due to the limited available spectral acquisition time per CSI voxel. A qualitative comparison however, shows a better spectral quality as well as a sharper peak, with the full width at half maximum values being 0.10 ppm for PRESS and 0.19 ppm for the CSI spectrum shown in Fig. 2B.
In vivo measurements displayed in Fig. 3 show that the sequence can detect both pyruvate and lactate in the kidneys (Fig. 3A and D) as well as smaller quantities in muscle tissue and liver (Fig. 3B-C).
Conclusion
A PRESS sequence was extended to multiple voxels and tested with thermal 1H, hyperpolarized 13C phantoms and in vivo. Thermal phantom 1H experiments on water, ethanol, and canola oil and with hyperpolarized [1-13C]pyruvate showed that voxel sizes down to 1x1x1mm3 are accurately localized. The first in vivo measurement in a healthy mouse showed that MV-PRESS can detect metabolites with a high sensitivity, without restriction to a 2D slice. Especially in cases where multiple lesions are present that cannot be covered by a single CSI slice, this sequence could be applied. Furthermore the sequence can be extended to a desired number of voxels, with the only limitation that overlap between excitation and refocusing slices of all voxels has to be excluded. This is enforced by a self-written software tool and in conclusion also limits the possible number of voxels depending on their respective size. Since MV-PRESS enables high sensitivity quasi-3D static acquisition of NMR spectra, it might be specifically beneficial for hyperpolarized sensor molecules which do not require time-resolved acquisitions (e.g. for detecting pH or metal ions) or to detect metabolite ratios in cases for which low SNR conditions prevent a full 3D dynamic readout.Acknowledgements
We acknowledge support from Martin Grashei with in vivo imaging protocols and Sandra Sühnel in helping with animal experiments. This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Reasearch Foundation, Emmy Noether program, grant number 391523415).References
1. Ardenkjær-Larsen, J. H., Fridlund, B., Gram, A., Hansson, G., Hansson, L., Lerche, M. H., ... & Golman, K. (2003). Increase in signal-to-noise ratio of> 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences, 100(18), 10158-10163.2. Topping, G. J., Hundshammer, C., Nagel, L., Grashei, M., Aigner, M., Skinner, J. G., ... & Schilling, F. (2020). Acquisition strategies for spatially resolved magnetic resonance detection of hyperpolarized nuclei. Magnetic Resonance Materials in Physics, Biology and Medicine, 33(2), 221-256.
3. NMR group of the Institute of Scientific Instruments of the Czech Academy of Sciences (2020), GitHub Repository. https://github.com/isi-nmr/brukerapi-python
4. Döpfert. J (2014), GitHub Repository. https://github.com/jdoepfert/brukerMRI
Figures
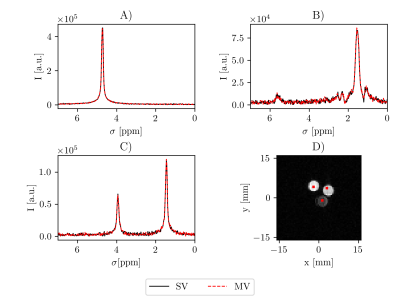
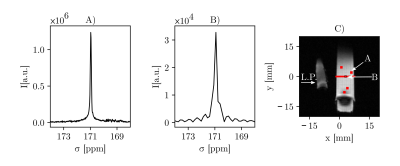
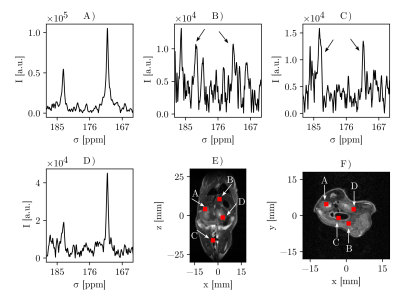
Figure 3: MV-PRESS spectra from an injection of a mouse with hyperpolarized [1-13C]pyruvate. Voxels are placed in the left kidney (A), liver (B), muscle (C), and right kidney (D) (line broadening 10 Hz). E) and F) show coronal and axial slices from a T2-weighted scan with the voxel positions projected onto the displayed slice.