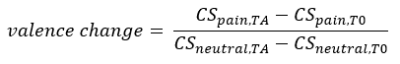1794
Resting-state functional connectivity predicts subsequent pain-related threat learning1Bingel-laboratory, Department of Neurology, University Hospital Essen, Essen, Germany, 2Laboratory of Predictive NeuroImaging, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany
Synopsis
Fear conditioning has a role in anxiety-disorders and the neurobiological correlates of it are not yet well understood. Therefore, we trained a machine learning predictive model on individual functional resting state connectivity data to predict the emotional aspects of fear conditioning. The model was found to predict individual pain-related threat learning measured by the change of valence with an explained variance of 24%-41%. These results highlight the potential of machine learning to enhance our understanding of fear conditioning.
Introduction
Previous results showed that deficits in fear conditioning contribute to the development and continuity of anxiety-disorders1. However, the biological correlates of the fear conditioning are not well understood in humans. The activity of several brain regions is accompanied with fear conditioning such as the anterior cingulate cortex, the amygdala and dorsolateral prefrontal cortex2. In addition, baseline functional connectivity is shaped by fear conditioning3-5. However, the link between resting functional connectivity and subsequent fear conditioning performance are not well understood. Therefore, we aimed to investigate whether baseline resting connectivity could be predictive on subsequent fear conditioning. A machine learning predictive model was developed on resting-state connectivity data to predict individual differences in pain-related threat learning.Methods
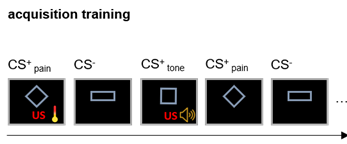
Participants (n=25) underwent a resting-state fMRI and a subsequent well established differential conditioning paradigm which involved two unconditioned stimuli: an unpleasant tone and painful heat stimuli. Three geometrical figures served as conditioned stimuli and one was followed by tone (CStone) and painful heat (CSpain). The participants reported how unpleasant was the CS for them (valence rating) and responses to the CSpain and CS- were used to calculate a differential measure of valence learning (Equation 1). During the acquisition phase participant learned the association between the unconditioned (pain) and conditioned stimuli (geometrical figure). We used this measure as the main target of our model. The fMRI data was pre-processed with our fixed pipeline (https://spisakt.github.io/RPN-signature/). The main steps of functional image pre-processing were the following: motion correction, despiking, nuissance regression (motion: Friston-24 and CompCor), bandpass filtering (0.008-0.08Hz) and scrubbing (threshold: framewise displacement >0.15mm). Participants were excluded based on excessive motion during the resting state measurement (mean FD > 0.15mm, percent of scrubbed volumes > 25%). Timeseries from 122 functional regions6 were used and the individual connectivity matrices based on partial correlation were calculated. The individual connectivity matrices were used as input features in our model. The model included a feature selection and L2 regularization step to predict individual valence rating change. Explained variance and correlation were used to investigate model performance both in a nested and non-nested leave-one-out cross validation (CV) frameworks.Results
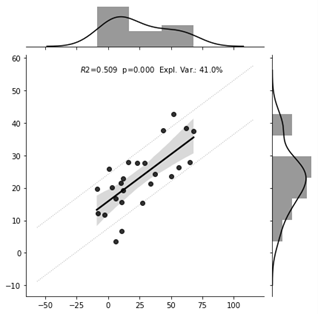
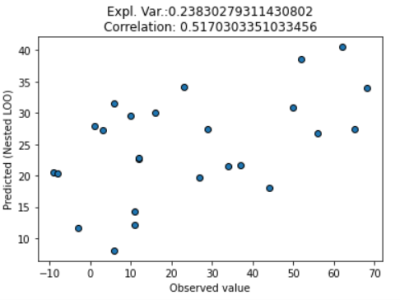
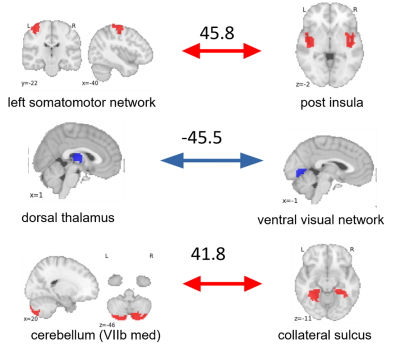
As a result of model training, 30 out of the 7503 connections were selected by the model. The prediction was driven by many regions which are not classically linked to fear conditioning. The top three connections in our model are the posterior insula-somatomotor network, thalamus-ventral visual network and cerebellar region VII-collateral sulcus (Figure 4). The model predicted valence changes with a root mean squared error of 17.9 and 20.3 on VAS100, and it could explain 41% and 24% of variance in the non-nested and in the nested CV, respectively. The correlation between the observed and predicted values were 0.71 and 0.52 for the non-nested and nested CV, respectively(Figure 2-3).Discussion
The unbiased estimation of our model performance (non-nested CV) suggests that our model can predict pain-related threat-learning measured by valence change. Therefore, resting functional connectivity has the potential to predict subsequent pain-related learning behavior. Connections between new brain regions emerged besides the classically involved brain regions and draw attention to not well investigated areas such as the cerebellum. Because of the limited number of participants, further validation of our model is essential to test its generalizability on unseen data.Conclusion
Delineating the resting connections which are related to pain-related threat learning might help us to better understand the neurobiological correlates of anxiety-related disorders. The emergence of new brain regions can deepen our understanding of the neurobiological correlates of threat-learning. Moreover, this model might be considered as a biomarker candidate of threat-learning.Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 316803389 – SFB 1280 and TRR 289 Treatment Expectation - Projektnummer 422744262.References
1. Lissek S, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005 Nov;43(11):1391-424. doi: 10.1016/j.brat.2004.10.007. PMID: 15885654.
2. Fullana, M. A. et al. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol. Psychiatry 21, 500–508 (2016).
3. Schultz, D. H., et al. Resting-state connectivity of the amygdala is altered following pavlovian fear conditioning. Front. Hum. Neurosci. 6, (2012).
4. Linnman, C., et al. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biol. Psychol. 89, 450–459 (2012).
5. Fraenz, C. et al. Fear learning sculpts functional brain connectivity at rest beyond the traditional fear network in humans. bioRxiv 2020.05.26.115840 (2020) doi:10.1101/2020.05.26.115840.
6. Urchs, S. et al. MIST: a multi-resolution parcellation of functional brain networks. MNI Open Res. 1, 3 (2017).
Figures