1784
Insights to the diffusion MRI ‘crossing fiber problem’: Characterizing the micro-structure in an MRI voxel using synchrotron radiation imaging1Technical University of Denmark, Kgs. Lyngby, Denmark, 2Danish Research Centre for Magnetic Resonance, Hvidovre, Denmark, 3European Synchrotron and Radiation Facility, Grenoble, France
Synopsis
Complex fiber regions where multiple tracts and bundles intersect are challenging to study with diffusion weighted MRI (DWI), due to the anatomical variation and 3D complexity within the volume covered by the measure voxel. Using x-ray nano-holotomography (XNH) we have been able to obtain and analyze a volume from a complex fiber region and make a comparison against DWI measurements from the same location of the same ex-vivo monkey brain. We derive comparable micro-structural features in the form of orientation distributions and anisotropy measures and show strong connections between them.
Introduction
Complex fiber regions where multiple tracts and bundles intersect are challenging to study with diffusion weighted MRI (DWI), due to the vast anatomical variation and 3D complexity within the volume covered by the measure voxel. This is colloquially known as the ‘crossing fiber problem’ [1]. Crossing fiber geometries or complex micro-structure exist in most of white matter DWI voxels, and can introduce bias into various DWI techniques, e.g. tractography and microstructure imaging techniques, if not taken into account.The diffusion tensor imaging (DTI) [2] is well-known to suffer from the ‘crossing fiber problem’, both with regards to estimating the fractional anisotropy (FA) and dominant orientations. Techniques such as Constrained Spherical Decomposition (CSD) [3] are widely agreed to better capture the orientation content when multiple fiber bundles exist. The micro-FA [4,5,6], on the other hand, measures the anisotropy of compartments within complex voxels independently of their fiber orientated distribution.
The crossing fiber problem naturally depends on the image resolution [1]. Schilling et al. found that DWI based measures were still influenced by complex micro-structure even in MRI voxel sizes of 32 microns. As such, we find that there is still a need to characterize the microstructure of a complex MRI voxel in sufficient resolution to observe individual axons in 3D, to obtain a full understanding of the micro-structural complexity. It is important that the volume field-of-view is close to the voxel size of the MRI, such that the anatomical information is in correspondence.
Synchrotron facilities provide options for 3D tomography while balancing the inverse relationship of field-of-view (FOV) and resolution trade-off. Using x-ray nano-holotomography (XNH) we have been able to obtain and analyze a volume from a complex fiber region and make a comparison against DWI measurements from the same location of the same ex-vivo monkey brain.
Methods
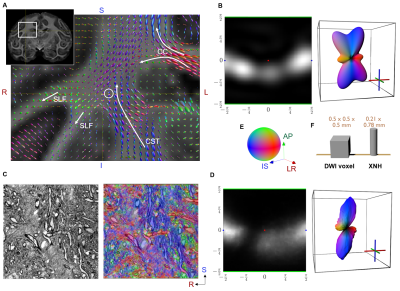
Data Acquisition: In this study, we include data from an ex-vivo vervet monkey brain. Full details are given in Andersson et al. [7]. In short, the whole brain was scanned with diffusion MRI (isotropic voxel size 0.5 mm). A biopsy extracted from a complex region where the Cortical Spinal Tract (CST), Corpus Callosum (CC) and superior-longitudinal fasciculus (SLF) intersect was scanned using XNH at the European Synchrotron and Radiation Facility (ESRF). The obtained FOV was 0.78 x 0.21 x 0.21 mm in a voxel resolution of 100 nm. For comparison, a biopsy from within the Corpus Callosum (CC), which is a straight fiber region, was also obtained.DWI Analysis: In all voxels of the brain, we perform diffusion tensor fitting and Constrained Spherical Deconvolution (CSD) [8] to estimate the fiber orientation distributions (FOD) (Figure 1A and 1B).
XNH Analysis: We perform a 3D structure tensor (ST) estimation [9] in each voxel within the synchrotron field-of-view (FOV). Using an eigen decomposition, we extract the principal direction vectors and associated eigenvalues. The eigenvalues are inverted and normalized, which in effect converts the ST to a diffusion-like tensor, to make comparisons to DWI more intuitive. The parameters for the ST estimation are adaptively chosen for each voxel within a pre-selected range (patch sizes ranging between 9.2 – 2 microns), such that the fractional anisotropy of the resulting tensor is maximized. This scale space parameter selection ensure that axons of different sizes give similar tensor outputs and tries to minimize the influence of the complex micro-structure environment on our analysis.
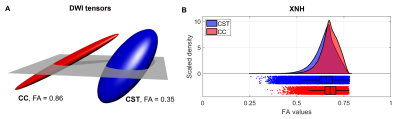
To create a FOD for the whole XNH volume, we sample the principal direction vector in each voxel on a spherical histogram (Figure 1D). The statistics of the tensor fractional anisotropy (FA) from each voxel are collected and a kernel density estimation is used to show the distributions (Figure 2B).
Results and Discussion
Orientation distributions: The FODs from DWI and XNH are compared in Figure 1B and 1D and are seen to be in good agreement. The CSD is able to correctly capture the orientation distribution even in this complex environment. The discrepancies are likely the consequence of not having the exact same voxel/FOV size and the uncertainty in co-registration.Anisotropy: The FA profiles derived from XNH volumes are shown in Figure 2B. They are highly similar, with the complex tissue being slightly less anisotropic. This demonstrates that at high enough image resolution, it is feasible to estimate the ST locally in individual axons with minimal influence from fiber dispersion and neighboring axons. Notable, the ST patch size covers a similar volume as explored by diffusion spins in MRI. Therefore, our ST-FA results resembles the idea of the micro-tensor domains in DWI techniques presented in Andersen et al. [Andersen20]. Although the two techniques are based on different contrast mechanisms to detect tissue anisotropy, it seems plausible that one can serve to validate the other in future studies.
Conclusion
We take the first steps in validating DWI-based measures in micro-structural complex regions. We have resolved a volume corresponding approximately to an MRI voxel in ultra-high resolution. Through structure tensor analysis we derive comparable micro-structural features in the form of orientation distributions and anisotropy measures. We demonstrate good agreement with orientations derived with CSD. Further, we show that anisotropy measured at this high resolution is the similar in both straight and complex regions.Acknowledgements
HMK and MA were supported by the Capital Region of Denmark Research Foundation (grant number: A5657) (PI:TD)References
[1] K. Schilling, Y. Gao, V. Janve, I. Stepniewska, B.A Landman, A.W. Anderson. Can increased spatial resolution solve the crossing fiber problem for diffusion MRI? NMR in Biomedicine, 2017.
[2] S. Mori, J. Zhang. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 2006.
[3] J. D. Tournier, F. Calamante, A. Connelly. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage, 2007.
[4] K. W. Andersen, S. Lasič, H. Lundell, M. Nilsson, D. Topgaard, F. Sellebjerg, F. Szczepankiewicz, H. R. Siebner, M. Blinkenberg, T. B. Dyrby. Disentangling white-matter damage from physiological fibre orientation dispersion in multiple sclerosis. Brain Communications, 2020.
[5] S.N. Jespersen, H. Lundell, C. K. Sønderby, T.B. Dyrby. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed, 2013.
[6] S. Lasič, F. Szczepankiewicz, S. Eriksson, M. Nilsson and D. Topgaard. Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Frontiers in Physics, 2014.
[7] M. Andersson, H.M. Kjer, J. Rafael-Patino, A. Pacureanu, B. Pakkenberg, J.P. Thiran, M. Ptito, M. Bech, A.B. Dahl, V.A. Dahl and T.B. Dyrby. Axon morphology is modulated by the local environment and impacts the noninvasive investigation of its structure–function relationship. Proceedings of the National Academy of Sciences, 2020.
[8] J.D. Tournier, R.E. Smith, D. Raffelt, R. Tabbara, T. Dhollander, M. Pietsch, D. Christiaens, B. Jeurissen, C.H. Yeh, and A. Connelly. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019.
[9] A.R. Khan, A. Cornea, L.A. Leigland, S.G. Kohama, S.N. Jespersen, C.D. Kroenke. 3D structure tensor analysis of light microscopy data for validating diffusion MRI. Neuroimage, 2015.
Figures