1783
Ultra-Short Echo Time 31P 3D MRSI at 3T with Novel Rosette k-space Trajectory1School of Health Sciences, Purdue University, West Lafayette, IN, United States, 2Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 3Department of Radiology, Medical Center, University of Freiburg, Freiburg, Germany, 4Department of Radiology, University of California, Los Angeles, CA, United States, 5Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 6Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
Feasibility of a novel, rosette trajectory UTE (70 μs) 3D 31P MRSI sequence is tested at 3T in phantoms and in upper leg scan with healthy subject. Braino phantom, proton resolution phantom, and highly concentrated 31P phantom test demonstrated proper reconstruction and metabolite localization. In vivo calculations of acquired PCr and β-ATP signals showed competitive SNR across 20 voxels of interest. With further acceleration, the sequence may serve as a superior alternative to conventional weighted 3D MRSI, allowing for greater SNR or resolution in briefer times. Notably, this unprecedentedly short UTE 31P MRSI intrinsically avoids baseline and phasing issues.
Introduction
Phosphorus-31 (31P) MRS can be an invaluable tool for probing in vivo levels of metabolites such as phosphocreatine (PCr), inorganic phosphate (Pi), and ATP (beta, alpha, and gamma). These 31P metabolites can be highly indicative of tissue pH, rest-exercise-recovery dynamics in skeletal muscle, and even non-invasive prognosis in liver disease1. However, due to short T2 relaxation time and low abundance in tissue, 31P MRS experiments are burdened by extremely low in vivo relative sensitivity; thus, it can be difficult to achieve both sufficient SNR and high resolution in a “patient-friendly” length scan2. To address this problem, a novel, rosette trajectory, ultra-short echo time (UTE, 70 μs) 31P 3D MRSI sequence with a spectral bandwidth of 2.2 kHz was implemented and tested both in a phantom and healthy volunteer.Methods
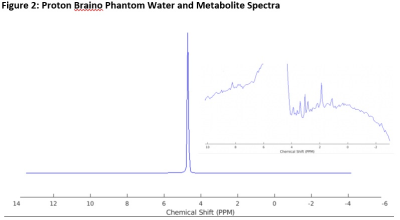
All data acquisition occurred using the 3T Siemens Prisma scanner at the Purdue MRI Facility.The magnetic resonance spectroscopic imaging (MRSI) with an ultra-short echo time (UTE) was developed based on a rosette k-space pattern. The rosette trajectory is well known for the multiple crossings of k-space origin, which leads to smooth transitions in temporal repetitions for spectra3. The parameters for the in vivo rosette-based MRSI sequence are as follows: field of view (FOV) = 480 mm3, matrix size = 24x24x24, spectral bandwidth = 2.2 kHz, temporal points = 256, TE = 70 microseconds, TR = 350 ms, 1444 petals for 3D k-space coverage. The rosette-based MRSI sequence was tested initially with proton resonance frequency and 20-channel receive-only head coil on a GE Braino phantom with TE = 20 μs, FOV = 240 mm3.
Following these successful proof-of-concept experiments (Figures 1 and 2), the sequence was transitioned to 31P MRSI and a previously custom-built, dual-tuned 1H/31P Tx/Rx 8-channel volume coil (2 plates with 4 channels each). The signal localization phantom, constructed of a 10 L carboy (36x21x15 cm) and filled with a background solution of 15 mM potassium phosphate monobasic (Pi), 75 mm NaCl, and 0.04 g/L NiCl2, contained three distinct spherical compartments filled with extremely high concentrations of 31P metabolites; the three spheres contained solutions of 100 mM MPA (resonance ~34 ppm), 100 mM PPA (~20 ppm), and a mixture of 100 mM MPA and 50 mM Pi (~5 ppm) respectively. A 3-average, 27-minute acquisition again confirmed viable SNR and localization, prompting the move to in vivo.
Leg muscle was determined to be the most convenient choice for SNR measurements and MRSI metabolite maps. The volunteer (healthy adult male) was positioned feet-first and supine, upper thighs surrounded by the parallel plates of the same 8-channel coil as before. Aiming for as much in vivo SNR as possible in a reasonable amount of time, the acquisition was set to 4 averages (36 minutes).
Results
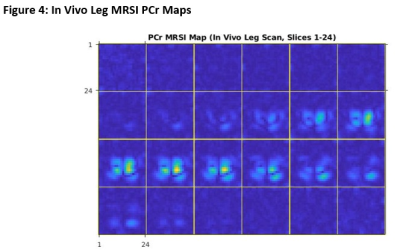
Figure 1 and Figure 2 illustrate the success of the rosette-based MRSI in proton phantoms prior to 31P experiments. The spherical shape is clearly discernible and the signal fairly uniform, with minimal amounts of ghosting outside the phantom volume. Typical 1H brain spectra is observable on the tail end of the water peak. As expected, the time between echoes is consistently equal to the inverse of the spectral bandwidth.Figure 3 showcases the sequence parameters (3A) one of the leg images (slice 15 of 24) and its corresponding PCr MRSI metabolite map (3B), and two example spectra from the chosen VOIs on this slice (3C). A table summarizing the mean SNRs (calculated by dividing the metabolite signal by RMSnoise,10-14 ppm) for both the PCr (0 ppm) and β-ATP (-16 ppm) peaks is also included (3D). Figure 4 showcases PCr MRSI maps successfully generated for both legs across all 24 slices (disappearing in between the legs and in slices outside the coil volume).
Discussion and Conclusion
The results show that our novel UTE (20 μs in proton, 70 μs in 31P) rosette trajectory MRSI sequence can correctly acquire and reconstruct both phantom and in vivo data with competitive SNR in reasonable amounts of time. Figure 3C especially highlights the utility of this record-short UTE for intrinsically avoiding phasing and baseline errors in spectra. Future acceleration of such a sequence could lead to significant advancements in SNR and resolution per minute of scan time, most notably in 31P (and other extremely fast T2 nuclei) MRS.Acknowledgements
Data acquisition was supported in part by NIH grant S10 OD012336 – 3T MRI Scanner dedicated to Life Sciences Research – and by the Indiana CTSI NIH grant UL1TR002529.
References
1Jeon MJ, Lee Y, Ahn S, et al. High resolution in vivo 31P-MRS of the liver: potential advantages in the assessment of non-alcoholic fatty liver disease. Acta Radiol. 2015;56(9):1051-1060.
2Meyerspeer M, Boesch C, Cameron D, et al. 31P magnetic resonance spectroscopy in skeletal muscle: Experts' consensus recommendations [published online ahead of print, 2020 Feb 10]. NMR Biomed. 2020;34(5):e4246.
3Noll DC. Multishot rosette trajectories for spectrally selective MR imaging. IEEE Trans Med Imaging. 1997;16(4):372-377.
Figures
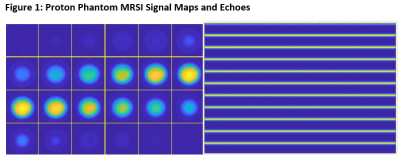
Left: MRSI signal intensity maps for spherical GE Braino phantom (all 1-24 slices).
Right: Plot of first 11 echoes (y-axis = kf*npetals = 11 * 1444). As expected, the distance between each echo is consistently equal to the inverse of the spectral bandwidth (1/SBW).
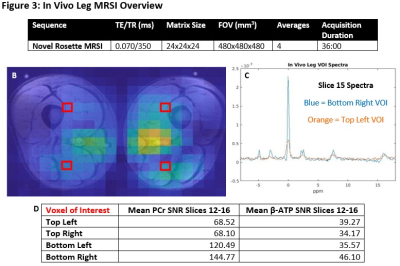
A: Sequence parameters.
B: PCr MRSI metabolite map (slice 15 of 24) overlaid on anatomical image.
C: Two example spectra from slice 15 chosen VOIs.
D: Summary of calculated SNRs for VOIs over slices 12-16.