1781
A Novel Acquisition Strategy for Volumetric Diffusion-Weighted Imaging using Variable Density TSE-CASPR1Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Diffusion weighted imaging (DWI) is a highly valuable MR imaging technique in the clinic. Single-shot EPI is the reference standard sequence for DWI due to its speed, however it suffers from severe distortion artifacts that can obscure potential pathologies. In this abstract, we present a novel 3D acquisition strategy that uses a fast spin echo readout and variable density sampling for volumetric DWI. The oversampled center region serves as navigator to correct phase errors. We present preliminary results in brains of healthy volunteers compared to the standard sequences used in DWI.
Introduction
Single-shot spin-echo EPI (SE-EPI) has become the reference standard acquisition for diffusion-weighted imaging (DWI), mainly because of their speed and low sensitivity to motion artifacts 1,2. However, EPI sequences suffer from several drawbacks including geometric distortions and signal pile-up in areas with large B0 inhomogeneities. Fast or turbo spin-echo (FSE/TSE) methods have been proposed as an alternative due to their increased robustness to B0 inhomogeneities 3. However, TSE-DWI are limited to 2D acquisitions, require long acquisition times, often suffer from motion related artifacts and require additional navigator information to account for induced phase errors 4. In this work, we present a novel acquisition strategy for volumetric DWI using a 3D multishot TSE based on variable density sampling with Cartesian acquisition using spiral profile ordering (VD-CASPR) 5.Theory and Methods
Conventional 3D TSE acquisitions normally follow a linear view order where echoes for each kz location are sequentially acquired before moving to the next kz location. With these ordering schemes, the center ky-kz profiles are only sampled once throughout the scan. We propose a VD-CASPR view ordering that samples the ky-kz profiles in a pseudo-spiral trajectory on a Cartesian grid. With this approach, a user-defined central region of the ky-kz space is fully sampled in each echo train, while the outer ky-kz space is undersampled in each echo train (Fig. 1a). At the end of the acquisition, the entire k-space is fully sampled, while the user-defined central region is oversampled by the number of shots (Fig. 1b). This fully sampled central region in each shot increases the signal to noise ratio (SNR) and also provides the navigator information that can be used for phase correction.Based on the field of view (FOV) and echo train length (ETL), the acquisition trajectory is calculated in such a way that the first N points of each ETL (profiles closest to the center of k-space, Ninner) are acquired in every shot. This in turn partly increases the total number of shots required to sample the entire k-space by a factor dependent on the ETL, Ninner, and the phase (Ny) and slice encoding (Nz).
The VD-CASPR sequence was combined with a phase-insensitive diffusion preparation and implemented on a 3T Ingenia scanner (Philips Healthcare, Eindhoven, the Netherlands). The sequence was initially optimized on a vendor-supplied resolution phantom. Subsequently, the sequence was evaluated in brains of 2 healthy volunteers under an approved IRB protocol. The acquisition parameters for 3D DWI with VD-CASPR were: TR/TE = 2500/75 ms, ETL = 96, acquired resolution = 2.5x2.5x3 mm3, FOV = 240x240x90 mm3, Ny = 109, Nz = 27, Ninner = 24, b = 0, 500, and 1000 s/mm2, scan time = 5:30 minutes. After acquisition, the raw data were exported for offline reconstruction in MATLAB (Mathworks Inc., Natick, Massachusetts) including phase correction. For this step, two different datasets were made for each shot – one containing the partially sampled k-space covering the entire ky-kz space; and the other containing only the oversampled Ninner center profiles (windowed dataset). Both k-spaces were Fourier transformed and the phase from the windowed dataset was subtracted from the original dataset in image space. Finally, the data were averaged across all shots to generate the final phase-corrected DW images. These were compared to standard DW images acquired using single-shot DW-EPI and DW-TSE sequences with similar acquisition parameters.
Results
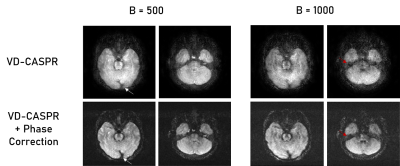
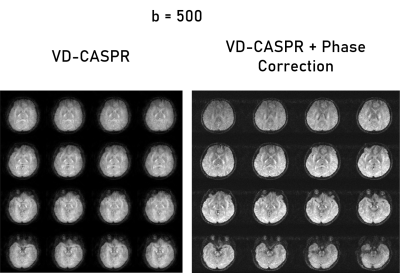
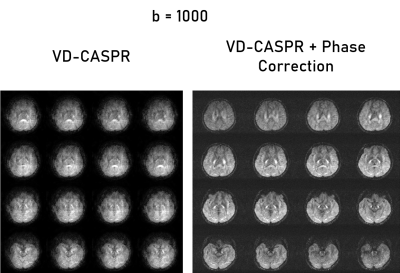
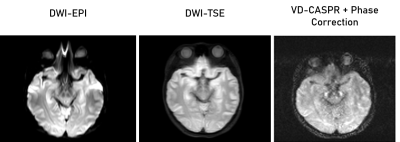
The VD-CASPR generated images with minimal artifacts at b = 0 s/mm2, but encountered severe artifacts at higher b-values, which were subsequently rectified using phase correction (Fig. 2). In the non-phase corrected images, there are several pile-up artifacts and blurring near tissue interfaces, which were resolved with the phase correction. Additionally, there is noticeable reduction of noise within the subject’s anatomy. Figures 3 and 4 show the improvements of the phase correction for VD-CASPR diffusion weighted images acquired with the same b-values for a different healthy subject. It is evident that phase-corrected VD-CASPR images display anatomical regions more clearly and without blurring. This is particularly appreciated at the higher b-values (e.g., b = 1000 s/mm2, Fig. 4), due to larger diffusion gradients. Lastly, compared to DW-EPI, VD-CASPR achieved improved robustness to geometric distortions and were comparable to DW-TSE acquisition (Fig. 5).Discussion and Conclusion
To our knowledge, this is the first demonstration of a volumetric DW image using TSE acquisition. Our proposed phase-corrected VD-CASPR sampling technique demonstrated high-quality diffusion weighted images, and achieves improved robustness to B0 inhomogeneities compared to DW-EPI. The image quality with VD-CASPR is currently sub-optimal compared to DW-TSE acquisitions, however, the slice thickness with 3D VD-CASPR were acquired at 3 mm and reconstructed to 1.5 mm, while the slice thickness for DW-TSE were 5 mm. Further optimization of the acquisition and reconstruction protocol can enable robust volumetric DW images in anatomies with increased susceptibility to B0 inhomogeneities such as spine and in body imaging.Acknowledgements
This work was partly supported by the Cancer Prevention and Research Institute of Texas (CPRIT) grant RP190049.References
1. Anderson, A. W. and J. C. Gore (1994). “Analysis and correction of motion artifacts in diffusion weighted imaging.” Magn Reson Med 32(3): 379-387.
2. Turner, R., et al. (1990). “Echo-planar imaging of intravoxel incoherent motion.” Radiology 177(2): 407-414.
3. Alsop, D. C. (1997). "Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans." Magn Reson Med 38(4): 527-533.
4. Ordidge, R. J., et al. (1994). “Correction of motional artifacts in diffusion-weighted MR images using navigator echoes.” Magn Reson Imaging 12(3): 455-460.
5. Greer, JS et al. Robust pCASL perfusion imaging using a 3D Cartesian acquisition with spiral profile reordering (CASPR). MRM 2019; 82(5):1713-1724.
Figures