1780
Evaluating b-value combinations on IVIM-DKI analysis with parametric reconstruction method in pancreatic carcinoma: A digital phantom study1Centre for Biomedical Engineering, Indian Institute of Technology Delhi, Navi Mumbai, India, 2Department of Radiodiagnosis, All India Institute of Medical Sciences Delhi, New Delhi, India, 3Department of Physics, Indian Institute of Technology Delhi, New Delhi, India, 4Sydney Imaging and School of Biomedical Engineering, University of Sydney, Sydney, Australia, 5Indian Institute of Technology Delhi, New Delhi, India, 6Department of Biomedical Engineering, All India Institute of Medical Sciences Delhi, New Delhi, India
Synopsis
Optimization of b-values in IVIM-DKI is required for parameter estimation accuracy. IVIM-DKI model with Total Variation correction approach was employed in this study, which avoids abrupt changes in parameter values and eliminates non-physiological heterogeneity in maps. In pancreatic cancer, different b-value combinations were used: 4,6,8,10, and 13b-values. In terms of accurate parameter estimations and comparable qualitative maps, 8-13 b-values exhibited a similar trend; in contrast, 4b-values indicated inaccurate and noisy maps. 8b-values can be employed in pancreatic cancer with a constrained technique for a shorter acquisition process and reduced noise in parameter maps at low SNR.
Introduction
Intravoxel incoherent motion combined with diffusion kurtosis imaging(IVIM-DKI) provides simultaneous insight into diffusion and perfusion data adjusted for non-Gaussian water movement distribution1–3. When compared to other diffusion-weighted imaging(DWI) models, the IVIM-DKI model accurately represents tumor heterogeneity1. While modeling the tissue environment, the main component is to select an optimal combination of b-values and model for robust and efficient parameter estimations. Optimization of b-values can also aid in lowering acquisition time, which is viable for scans of abdominal, whole-body, and critically-ill patients or pediatric populations. IVIM and DKI have demonstrated promise in distinguishing pancreatic cancer from other pancreatic masses4. We investigated the impact of b-value combinations on IVIM-DKI analysis with constrained method employing total variational(TV) penalty function with digital phantoms of pancreatic carcinomas.Methods
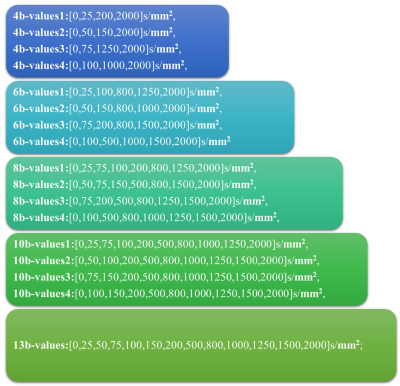
b-value combination: Figure1 shows b-value combinations utilized, where 4, 6, 8, 10 number of b-values and four different combinations of b-values were used for each set, and 13 b-values were used as best-case reference(as per clinical routine)6-8.Simulation: Four simulations were carried out by varying one IVIM-DKI parameter a time and keeping all other parameters constant:
• Simulation I:D =[0.7,1,1.3,1.6,1.9,2.2]x10-3 mm2/s;D*=16x10-3 mm2/s;f=0.15;k = 0.8
• Simulation I:D* =[7,10,13,16,19,22] x10-3 mm2/s;D=1.6x10-3 mm2/s;f=0.15;k=0.8
• Simulation III:f =[0.05,0.1,0.15,0.2,0.25,0.3];D = 1.6x10-3 mm2/s;D*=16x10-3 mm2/s;k=0.8
• Simulation IV:k=[0.4 0.6 0.8 1 1.2 1.4];D=1.6x10-3 mm2/s;D*=16x10-3 mm2/s;f=0.15
Based on a literature search, the values of parameters were chosen to include pancreatic carcinomas and healthy pancreas4,5. White Gaussian noise with varying variance was used to generate IVIM-DKI digital phantoms with SNRs of 15,30,40,50, and 60(low to high SNR).
IVIM-DKI Image analysis: Parameter estimation was performed using an in-house build toolbox in MATLAB. All data were processed using both standard IVIM-DKI model(traditional model)1 and IVIM-DKI model with TV(advanced model)6. Non-least linear square optimization was used keeping four free parameters(D,D*,f,k) for both traditional and advanced models. In the advanced model, 3D TV was used to minimize TV of the whole image stack at once.
Parameter error estimation in Simulation data: As true values are known in simulations, RRMSE was calculated for each parameter(Drrmse,D*rrmse, f rrmse, and krrmse) in percentage:
$$X_{r r m s e}=\frac{\sqrt{\frac{\sum\left(X-X^{\prime}\right)^{2}}{N}}}{\overline{X}}\times 100$$
X' is estimated parameter values, and X is reference parameter values, and N is the total number of elements in reference map}$$. Total parameter RRMSE was calculated by taking the average of all parameters’ RRMSE.
Results
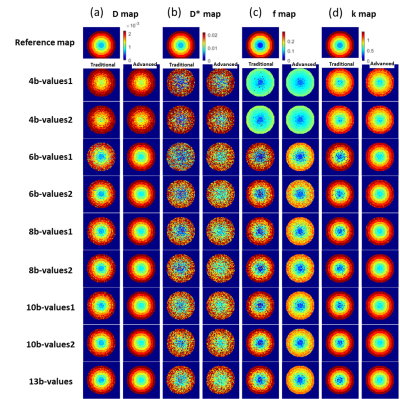
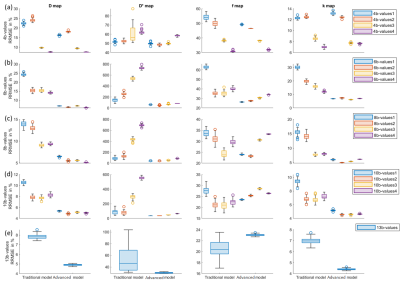
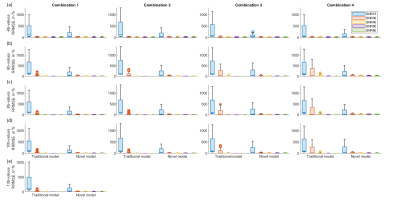
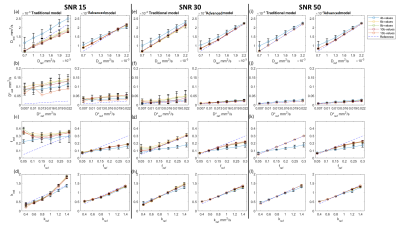
Figure2 presents the parameter reconstruction at SNR30(moderate SNR), showing an advanced model for any b-value combination presents a better quality of parameter maps than the traditional model. Figure3 shows there was a significant reduction by 5-89% in parameter estimation error by the advanced model at SNR 30. In D, f, and k map, lower RRMSE by 22 to 51% was observed for b-value combination 3 and 4 as compared to 1 and 2 combinations for 4-10 numbers of b-value. In D*, RRMSE increases with b-value combination 1 to 4.Overall, total error in parameter estimation of advanced model decreased by 8-87% at SNR15 and 30, but the error was observed to increase by 7-50% at high SNRs, SNR40, 50, and 60 as shown in figure4.
Figure5 shows a comparison between reference and estimated parameter values, at 4b-values combination; where parameters were highly overestimated or underestimated. Overall, the advanced model consistently showed a low standard deviation of estimated parameter values at all SNR. For D at all SNR using 4b-value combinations were overestimated by both models; whereas lower k values simulated were overestimated using advanced model. D* estimated values by traditional model were highly varied from reference at all combinations. High simulated values of f parameter were found to be underestimated by the advanced model with all b-value combinations at all SNR.
Discussion
B-value optimization allows for precise quantification of diffusion and perfusion metrics acquired from IVIM-DKI analysis. Except for head and neck cancer7, no literature studies have optimized the acquisition strategy for IVIM-DKI analysis in pancreatic cancer. The advanced model successfully reduces estimation error at low SNR(15 and 30) and SNR40 for 6 and 8b-value combinations, but estimation error was increased at high SNR. Use of 4b-values combinations might result in substantial inaccuracy due to parameter under-/over-estimation at any SNR value, particularly with the D* parameter. A few numbers of low b-values ranging from 0-75 s/mm2 with either a traditional or advanced model can be employed for reliable D* measurement. The estimate of k was unaffected by any b-value numbers, combinations, or SNR. However, D and f, need greater number of b-values for optimization routine. 8b-values with advanced model and 13b-values combinations with traditional model showed comparable accuracy in parameter estimations, also shown in previous studies with IVIM model8. Thus, a similar scheme can be widely adopted for clinical routine.Conclusion
Parametric reconstruction utilizing the IVIM-DKI model was carried out using different b-value combinations. The advanced model was resilient to b-value combinations and enhanced quality of all parameter maps at low SNR. Acquisition of 8 b-values using advanced model produced comparable accuracy as 13b-values while taking shorter acquisition time.Acknowledgements
This study was supported by IIT Delhi and AIIMS Delhi. AVM was supported by research fellowship fund from Ministry of Human Resource Development, Government of India.References
1. Lu, Y. et al. Extension of the intravoxel incoherent motion model to non‐gaussian diffusion in head and neck cancer. J. Magn. Reson. Imaging 36, 1088–1096 (2012).
2. Le Bihan, D. et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505 (1988).
3. Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440 (2005).
4. Kim, B. et al. Intravoxel incoherent motion diffusion-weighted imaging of the pancreas: Characterization of benign and malignant pancreatic pathologies. J. Magn. Reson. Imaging 45, 260–269 (2017).
5. Klau, M. et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest. Radiol. 46, 57–63 (2011).
6. Malagi, A. V. et al. IVIM–DKI for differentiation between prostate cancer and benign prostatic hyperplasia: comparison of 1.5 T vs. 3 T MRI. Magn. Reson. Mater. Physics, Biol. Med. (2021). doi:10.1007/s10334-021-00932-1
7. Sijtsema, N. D. et al. An optimal acquisition and post‐processing pipeline for hybrid IVIM‐DKI in head and neck. Magn. Reson. Med. (2020). doi:https://doi.org/10.1002/mrm.28461
8. Malagi, A. V., Das, C. J., Khare, K., Calamante, F. & Mehndiratta, A. Effect of combination and number of b values in IVIM analysis with post-processing methodology: simulation and clinical study. Magn. Reson. Mater. Physics, Biol. Med. 32, 519–527 (2019).
Figures