1777
Clinical evaluation of scout accelerated motion estimation and reduction (SAMER)1Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Siemens Medical Solutions, Malvern, PA, United States, 6Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Patient motion can degrade diagnostic image quality to the point that scans must be re-acquired, or patients called back. In this work, we performed a systematic clinical evaluation of SAMER, a highly efficient retrospective motion mitigation approach. 62 patients from emergency and inpatient care settings were scanned at 3T, and 14 cases were identified with a range of patient motion. Two neuro-radiologists performed blinded review of the images before and after motion correction, rating the images for motion severity. In 13 of the 14 cases, reduced motion severity and improved visualization of anatomy/pathology was obtained after SAMER motion mitigation.
Introduction
Patient motion during the 2 to 5 minutes typically used to acquire an MR image volume causes the familiar “motion artifacts” that degrade diagnostic utility, often to the point that scans must be re-acquired, or the patient called back for a repeat scan [1]. A recent study at a US hospital found that 20% of MR scans were repeated due to patient motion, including 29% of inpatient and/or emergency department scans [2]. In the worst case, the radiologist simply lives with or “reads through” the artifacts, possibly missing diagnostic information [3].Many methods to detect and correct for motion in MRI have been explored [4], but few have gained traction in the clinical setting. Alternatives are few and may be to the detriment of the patient, including performing MRI under anesthesia, a procedure that increases risk, examination duration and cost [5]. In this work, we performed an evaluation of SAMER [6], a recently proposed retrospective motion-correction approach that facilitates multi-scale motion estimation by leveraging optimized data acquisition orderings and an ultra-fast scout prior. The motion mitigation performance of SAMER was evaluated by neuroradiologists on 3D T1-weighted brain MR images acquired on inpatients and emergency patients upon clinical deployment at Massachusetts General Hospital.
Methods
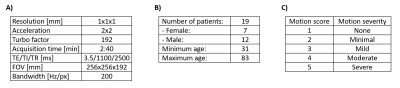
This single-center prospective study was approved by the IRB and conducted in accordance with HIPAA guidelines for research. From August-October 2021, clinical evaluation data were acquired from 62 patients in emergency and inpatient care settings on 3T systems (MAGNETOM Skyra and MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 20-channel head-neck coil. The imaging protocol included an R=4-fold accelerated T1-weighted MPRAGE sequence (Fig. 1A), which was acquired using a custom linear+checkered sequence reordering [6]. This sequence was incorporated into routine brain MRI protocols without contrast and with/without contrast in order to assess the performance of motion correction on noncontrast and contrast-enhanced exams. Retrospective reconstructions of the MPRAGE data were performed with and without SAMER motion correction. The 62 reconstructions without motion correction were screened for the presence of motion by an independent rater, and 14 relevant cases were identified. Details on the patient population are summarized in Fig. 1B.All motion affected cases were visually reviewed by two neuroradiologists (10 and 3 years of experience) in a blinded manner. A graded 5-tier scale (Fig. 1C, [2]) was used to measure the impact of motion artifacts on diagnostic image quality. Discrepancies in the two neuroradiologists’ scores were adjudicated by a third neuroradiologist (9 years of experience).
Results
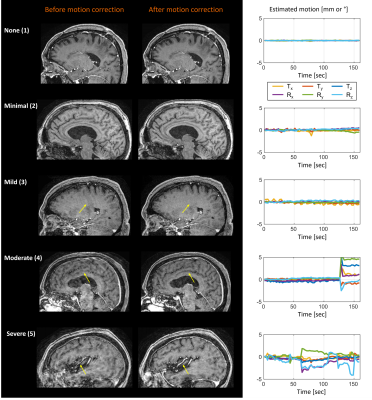
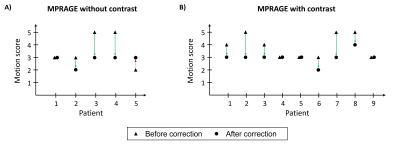
Figure 2 shows representative cases of the motion severity scale with none (1), minimal (2), mild (3), moderate (4) and severe (5) motion. The SAMER motion mitigated images are shown alongside the predicted motion time course (6 degree of freedom estimate of the rigid body motion parameters).Figure 3 shows the adjudicated motion scores before and after correction. In 13 of 14 cases with patient motion, SAMER produced improved scores. In the outlier case (non-contrast T1-weighted MRI, patient 5), the SAMER reconstruction led to a slightly worse image quality (increased from minimal to mild motion). In 6 of 7 cases with moderate or severe motion, only mild motion artifacts were present after the correction.
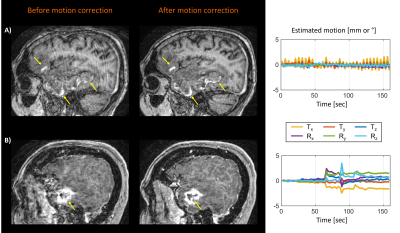
Figure 4 shows representative cases demonstrating improved visualization of pathology after motion correction in both pre- and post-contrast MPRAGE scans.
Discussion
We have deployed and evaluated SAMER motion correction in an inpatient clinical setting, where motion artifacts are a frequent clinical problem. Using a predefined radiologist scoring system, SAMER reduced the impact of patient motion in 93% (13 of 14) of the examined cases. In all but one case of moderate/severe motion, only mild motion artifacts were present after the correction. Our pilot study indicates that SAMER improves diagnostic image quality across a broad range of pathology, including cases in which motion artifacts likely obscured the visualization of key findings.In one case, model errors led to slightly reduced image quality after the correction. However, retrospective techniques such as SAMER allow for the standard image reconstruction to be performed and quantitatively compared alongside the motion-modeled solution. In future workflows, the cleaner image could be automatically determined and provided for clinical interpretation, thereby offering alternatives to radiologists and radiologic technologists seeking to optimize image quality against the competing demands of scan time and patient comfort.
Acknowledgements
This work was supported by research grants from the National Institutes of Health (grant no. P41-EB030006) and Siemens Healthineers.References
[1] R. B. van Heeswijk, G. Bonanno, S. Coppo, A. Coristine, T. Kober, and M. Stuber, “Motion compensation strategies in magnetic resonance imaging,” Crit. Rev. Biomed. Eng., vol. 40, no. 2, pp. 99–119, 2012.
[2] J. B. Andre et al., “Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations,” J. Am. Coll. Radiol., vol. 12, no. 7, pp. 689–695, 2015.
[3] M. Reuter, M. D. Tisdall, A. Qureshi, R. L. Buckner, A. J. W. van der Kouwe, and B. Fischl, “Head motion during MRI acquisition reduces gray matter volume and thickness estimates,” Neuroimage, vol. 107, pp. 107–115, 2015.
[4] M. Zaitsev, J. Maclaren, and M. Herbst, “Motion artifacts in MRI: A complex problem with many partial solutions,” J. Magn. Reson. Imaging, vol. 42, no. 4, pp. 887–901, 2015.
[5] S. A. Vanderby, P. S. Babyn, M. W. Carter, S. M. Jewell, and P. D. McKeever, “Effect of anesthesia and sedation on pediatric MR imaging patient flow.,” Radiology, vol. 256, no. 1, pp. 229–37, 2010.
[6] D. Polak et al., “Scout accelerated motion estimation and reduction (SAMER),” Magn. Reson. Med., vol. n/a, no. n/a.
[7] A. Deistung, A. Schäfer, F. Schweser, U. Biedermann, R. Turner, and J. R. Reichenbach, “Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength,” Neuroimage, vol. 65, pp. 299–314, 2013.
Figures