1776
A comparison of tractography and fMRI pre-surgical planning approaches with intraoperative mapping-based validation1Imaging and pathology, Translational MRI, KU Leuven, Leuven, Belgium, 2Department of Neurosciences, KU Leuven, Leuven Brain Institute, Leuven, Belgium, 3Department of Neurosciences, Neuropsychiatry, KU Leuven, Leuven, Belgium, 4Geriatric Psychiatry, University Psychiatric Center (UPC) - Leuven, Leuven, Belgium, 5Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium, 6Department of Radiology, University Hospitals Leuven, Leuven, Belgium, 7Department of Neurosciences, Research Group Experimental Neurosurgery and Neuroanatomy, KU Leuven, Leuven, Belgium, 8Department of Neurosciences, Laboratory for cognitive neurology, KU Leuven, Leuven, Belgium
Synopsis
Accurate presurgical brain mapping enables preoperative risk assessment and intraoperative guidance to minimize postoperative deficits. Here we compare mapping accuracy of task-based fMRI (tbfMRI), BOLD and Functionnectome resting state fMRI (rsfMRI), DTI and constrained spherical deconvolution (CSD)-based tractography in 21 preoperative neurosurgical patients using intraoperative electrical stimulation (DES) as the ground truth for functional mapping. Accuracy was estimated based on minimum distance between MRI-based mapping and positive DES coordinates. We report that CSD outperforms DTI, and rsfMRI performs similarly to tbfMRI using DES. This demonstrates the potential benefits of using CSD and rsfMRI in clinical practice.
Introduction
Presurgical brain mapping enables preoperative risk assessment and is used for intraoperative guidance to minimize postoperative deficits 1–3. Presurgical MRI mapping includes structural imaging, task-based fMRI (tbfMRI) for functional mapping, and diffusion tensor imaging (DTI) 4–6 scans for white matter mapping with fiber assignment by continuous tracking (FACT) 7. TbfMRI requires adequate task-performance, which limits its applicability only to patients who are able to perform the task of interest 8,9. DTI FACT is known to suffer from inaccuracies in the presence of complex fiber architecture 10. However, these techniques remain the mainstay of clinical presurgical MRI brain mapping despite the availability of alternatives such as resting state fMRI (rsfMRI) 11, and constrained spherical deconvolution (CSD) 12 based tractography, which address these limitations.Here we evaluated the accuracy of a) CSD to FACT based tractography and b) tbfMRI to resting-state fMRI (rsfMRI) in preoperative neurosurgical patients. Intraoperative electrical cortical and subcortical stimulation (DES) represented the ground truth. Accuracy was estimated based on minimum distance between MRI-based mapping and positive DES coordinates.Methods
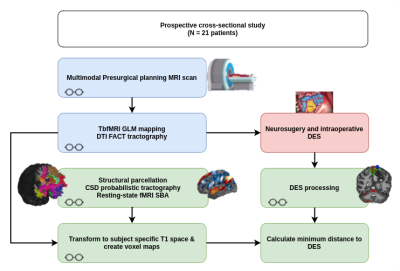
Multimodal presurgical MRI scanning (3T Philips) was performed on 21 surgically naïve patients who were prospectively recruited (14 tumors, and 7 epilepsy). All participating patients signed written informed consent and local ethics committee approval was acquired (S61759 - Leuven/Belgium). We acquired 3D high resolution structural images (1mm isotropic), tbfMRI (1.8x1.8x3.2 mm resolution, TR 1.5s, multiband factor 2) and rsfMRI (2.2 mm isotropic resolution, TR 0.9s, multiband factor 6) and diffusion MRI (2 mm isotropic resolution) (5 b0, 128d b1200, +/- 128d b2500). Patients underwent wake-up neurosurgery and DES (OSIRIS neurostimulator). DES points were considered positive if stimulation interfered with task performance or evoked a motor/sensory response. Spheres of 5 mm radius were constructed around positive coordinates and warped to subject-specific T1w space using FSL 13,14 and ANTs 15. TbfMRI data were processed using a general linear model in SPM12 16, DTI FACT tractograms of the corticospinal tract (CST) and arcuate fasciculus (AF) were manually generated using the Philips EWS clinical workstation, resulting tractograms were exported as voxel maps. Structural parcellation of the T1-weighted images was done using VBG 17, FreeSurfer 18 and MSBP 19. RsfMRI data was processed using the first level seed-to-voxel analysis in CONN 20 and the MSBP parcellation maps. CSD tractography was done using FWT 21. Preprocessed rsfMRI BOLD images were also processed using the region-wise Functionnectome 22 pipeline and processed with the first level seed-to-voxel in CONN. Tb-fMRI maps were thresholded at puncorrected < 0.05. All results were warped to the subject-specific T1w space using ANTs. Minimum distances were calculated between each DES point and the corresponding functional maps and/or tractograms voxel maps and used to represent mapping accuracy. A two-tailed paired t-test was used to compare DTI and CSD, and a one-way analysis of variance (ANOVA) with repeated measures was used to compare the fMRI results. Results were considered significant at p < 0.05. Figure 1 shows a schematic of data processing workflow.Results
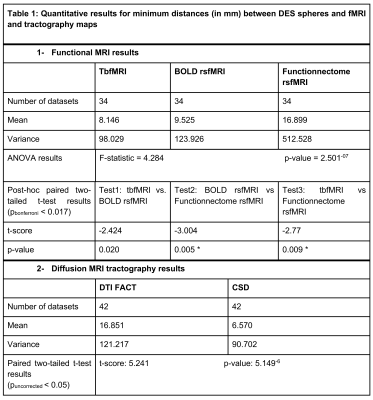
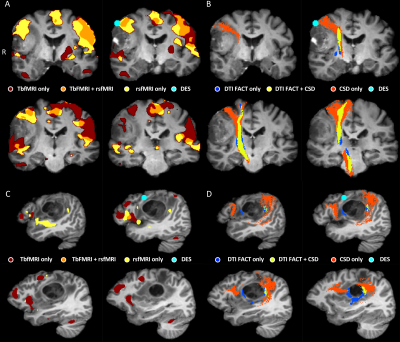
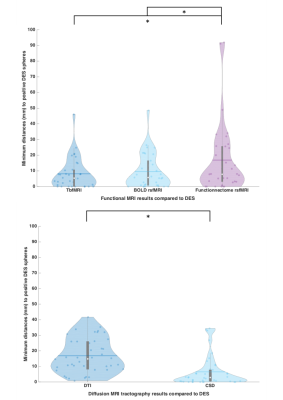
Table 1 shows summary statistics of the minimum distances and results of all tests. There was a significantly lower minimum distance between CSD fiber bundles and the DES coordinates compared to DTI bundles, indicating that CSD is more accurate than DTI FACT. There was a small difference in minimum distance between tbfMRI and rsfMRI that didn’t survive Bonferroni correction, and a significant difference between tbfMRI and Functionnetcome outputs. In both cases tbfMRI was found to be the most accurate. The differences found between DTI and CSD were the largest and most significant result. Demonstrative results for the SMN, and for the language network are shown in Figures 2 and 3.Discussion
We found a lower minimum distance between CSD tractograms and DES spheres demonstrating the added benefit of using CSD for fiber tracking compared to the clinical implementation of DTI FACT. This finding cannot be attributed solely to the CSD model, however, but also to the advanced diffusion preprocessing used 23–25. More accurate DTI results may be achievable with advanced preprocessing pipelines. In line with the literature 26–29, TbfMRI and BOLD rsfMRI showed only small and non-significant differences, confirming the reasonable accuracy of BOLD rsfMRI based localization of eloquent cortical regions. Additionally, rsfMRI can be used to investigate multiple RSNs simultaneously, making it more efficient than tbfMRI, which tends to be task-specific. This feature of rsfMRI may extend the scope of presurgical planning by additionally localizing higher-order cognitive and emotional processing networks 30 Functionnectome analysis results were less encouraging, however, this may be attributed to the presence of focal pathologies in our data, which changes the structural connectivity profile of the involved brain regions and violate the method’s assumptions. However, it does show promise and this study is the first to apply the Functionnectome approach to clinical data.Conclusion
In a neurosurgical setting, CSD outperforms DTI, and rsfMRI performs similarly to tbfMRI when using DES to localize functional brain regions. This demonstrates the potential benefits for using CSD and rsfMRI in clinical practice.Acknowledgements
No acknowledgement found.References
1. Kokkonen, S.-M. et al. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn. Reson. Imaging 27, 733–740 (2009).
2. Stippich, C. et al. Localizing and Lateralizing Language in Patients with Brain Tumors: Feasibility of Routine Preoperative Functional MR Imaging in 81 Consecutive Patients 1. Radiology 243, 828–836 (2007).
3. Sunaert, S. Presurgical planning for tumor resectioning. J. Magn. Reson. Imaging 23, 887–905 (2006).
4. Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).
5. Basser, P. J., Mattiello, J. & LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 103, 247–254 (1994).
6. Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A. & Chiro, G. D. Diffusion tensor MR imaging of the human brain. Radiology (1996) doi:10.1148/radiology.201.3.8939209.
7. Mori, S., Crain, B. J., Chacko, V. P. & van Zijl, P. C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999).
8. Manan, H. A., Franz, E. A. & Yahya, N. The utilisation of resting-state fMRI as a pre-operative mapping tool in patients with brain tumours in comparison to task-based fMRI and intraoperative mapping: A systematic review. Eur. J. Cancer Care (Engl.) 30, e13428 (2021).
9. Pujol, J. et al. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J. Neurosurg. 88, 863–869 (1998).
10. Jeurissen, B., Leemans, A., Tournier, J. D., Jones, D. K. & Sijbers, J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 34, 2747–2766 (2013).
11. Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI. https://pdfs.semanticscholar.org/693a/6e46be9b613ac5beab7313e1f0b51658dbe9.pdf (1995).
12. Tournier, J. D., Calamante, F., Gadian, D. G. & Connelly, A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage 23, 1176–1185 (2004).
13. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. NeuroImage 62, 782–790 (2012).
14. Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1, S208-219 (2004).
15. Tustison, N. J. et al. The ANTsX ecosystem for quantitative biological and medical imaging. Sci. Rep. 11, 9068 (2021).
16. Ashburner, J. et al. Statistical Parametric Mapping: The Analysis of Functional Brain Images. (2006).
17. Radwan, A. M. et al. Virtual brain grafting: Enabling whole brain parcellation in the presence of large lesions. NeuroImage 229, 117731 (2021).
18. Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
19. Tourbier, S., Aleman-Gomez, Y., Griffa, A., Bach Cuadra, M. & Hagmann, P. sebastientourbier/multiscalebrainparcellator: Multi-Scale Brain Parcellator v1.1.1. (Zenodo, 2019). doi:10.5281/zenodo.3627097.
20. Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
21. Radwan, A. et al. An atlas of white matter anatomy, its variability, and reproducibility based on Constrained Spherical Deconvolution of diffusion MRI. 2021.10.13.464139 https://www.biorxiv.org/content/10.1101/2021.10.13.464139v1 (2021) doi:10.1101/2021.10.13.464139.
22. Nozais, V., Forkel, S. J., Foulon, C., Petit, L. & Thiebaut de Schotten, M. Functionnectome as a framework to analyse the contribution of brain circuits to fMRI. Commun. Biol. 4, 1–12 (2021).
23. Brun, L., Pron, A., Sein, J., Deruelle, C. & Coulon, O. Diffusion MRI: Assessment of the Impact of Acquisition and Preprocessing Methods Using the BrainVISA-Diffuse Toolbox. Front. Neurosci. 13, 536 (2019).
24. Ressel, V., van Hedel, H. J. A., Scheer, I. & O’Gorman Tuura, R. Comparison of DTI analysis methods for clinical research: influence of pre-processing and tract selection methods. Eur. Radiol. Exp. 2, 33 (2018).
25. Roalf, D. R. et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. NeuroImage 125, 903–919 (2016).
26. Branco, P. et al. Resting-State Functional Magnetic Resonance Imaging for Language Preoperative Planning. Front. Hum. Neurosci. 10, 1–14 (2016).
27. Cochereau, J. et al. Comparison between resting state fMRI networks and responsive cortical stimulations in glioma patients. Hum. Brain Mapp. 37, 3721–3732 (2016).
28. Hacker, C. D., Roland, J. L., Kim, A. H., Shimony, J. S. & Leuthardt, E. C. Resting-state network mapping in neurosurgical practice: a review. Neurosurg. Focus 47, E15 (2019).
29. Kumar, V. A. et al. The role of resting-state functional MRI for clinical preoperative language mapping. Cancer Imaging 20, 47 (2020).
30. Catalino, M. P. et al. Mapping Cognitive and Emotional Networks in Neurosurgical Patients Using Resting-State Functional Magnetic Resonance Imaging. Neurosurg. Focus 48, E9 (2020).
Figures