1775
DWI of cholesteatomas: An initial assessment based on RESOLVE and SPEN MRI1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Sheba Medical Center, Ramat Gan, Israel, 3Azrieli National Center for Brain Imaging, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Cholesteatomas are destructive lesions growing in the middle ear, in need of periodic assessments. Diffusion weighted imaging (DWI) at 3T provides clear contrast of these growths, facilitating their detection. However, the close presence of air-tissue interfaces within the ear channel results in geometric distortions that have led to adoption of non-EPI based methods for these high field evaluations. Herein we present results from initial DWI experiments performed for imaging cholesteatomas, which point out the value of Spatiotemporal Encoding (SPEN) DWI methods as promising alternatives to retrieve high-contrast, high-definition images.
Introduction
Cholesteatoma is a collection of keratinous debris lined by stratified squamous epithelium trapped in the middle ear1,2. Growth of cholesteatomas is associated with displacement and eventual destruction of the ossicles and erosion of the middle ear cavity walls. The lesion causes a high signal intensity on diffusion weighted imaging (DWI), which therefore serves as the main mean for its detection3. Studies have shown that, due to the severe susceptibility artifacts arising at air-bone interfaces, EPI is less accurate than non-EPI-based DWI sequences for the detection and characterization of cholesteatomas –particularly at high fields4. Most commonly used for DW imaging of cholesteatoma are thus half-Fourier acquisition single-shot turbo-spin echo (HASTE) and BLADE/PROPELLER5. DW read-out (RO) segmented EPI (RESOLVE) has also been assessed for this end6–8. This study assesses the performance of the latter option against DWI based Spatiotemporal Encoding (SPEN) –another promising method for overcoming susceptibility induced image artifacts9– for cholesteatoma detection.The SPEN encoding used in this study relies on the application of a linearly swept adiabatic pulse in conjunction with a gradient, conferring spins with a phase having a quadratic dependence on position along the phase encoding (PE) direction. As a result of this, and by contrast to EPI techniques where immunity to field distortions is given by the minimum achievable echo spacing, SPEN can tune its immunity by changing the bandwidth (BW) chosen for the swept pulse –thereby adapting it as per the demands of the scanned object. Another salient feature of SPEN is its capability to reduce field-of-view (FOV) without suffering of folding, by tuning the extent of the frequency sweep to the region of interest. This allows one to reduce TE without sacrificing spatial resolution, simply by collecting fewer PE lines. SPEN can also be run in a full-refocusing mode providing additional robustness vs field inhomogeneities. Lastly, since for the case of interleaved acquisitions each of the individually collected segments will deliver aliasing-free images without recourse to multiple receivers, this allows one to correct for motion-induced phase variations between segments, as described elsewhere9.
Methods
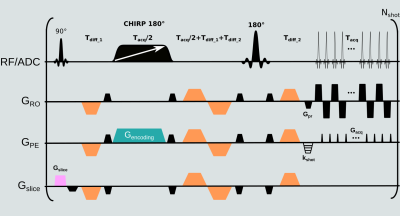
MRI were acquired at 3T on a Siemens Prisma. RESOLVE and SPEN DW images were acquired at 1.2⨉1.2⨉2 mm3 resolution, 14 slices with 30% gap both in transversal and coronal orientation, 4 diffusion encoding directions using b-values of 0 and 1000 s/mm2. For SPEN, 2 and 6 repetitions were acquired for the low and high b-values respectively; for RESOLVE one repetition per b-value was collected due to the higher number of interleaves of this experiment. SPEN’s FOV was 180⨉54 mm2, RESOLVE’s FOV was 230⨉230 mm2. For SPEN, partial RO FT sampling (75%) and 2 interleaved shots along the PE direction, were acquired. The PE BW was 3.6 kHz, TE 62 ms, TR 7 sec; experimental time 6 min 11 sec. The SPEN image processing pipeline introduced in Ref. 9 was translated into a Gadgetron reconstruction framework11,12, to provide real-time data processing. For RESOLVE, the RO axis was divided in 5 segments, GRAPPA acceleration of 2 was used, the effective PE BW was 5.6 kHz, TE 59 ms, TR 7 sec; experimental time was 2 min 45 sec.Seven patients suspected of having cholesteatoma were scanned prior to surgery, after obtaining suitable written consent. Out of the seven patients, surgery identified inflammation but no cholesteatoma in one of them, while for the rest cholesteatoma was confirmed during surgery. All except one of these 6 cholesteatoma cases was positively identified by both DWI sequences; for one of the cases both sequences (SPEN and RESOLVE) gave false negative diagnosis, missing a small cholesteatoma.
Results & Discussion
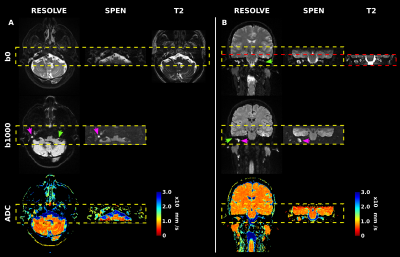
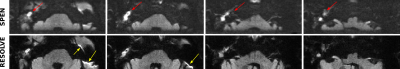
Representative images demonstrating SPEN’s robustness to susceptibility artifacts, are presented in Figure 2 for two patients. As evidenced by Fig. 2, both DW methods provided excellent cholesteatoma contrast in the b-weighted images, as well in ADC maps. Nonetheless, SPEN routinely displayed less distortions and allowed more faithful delineation of the shape of cholesteatoma for the examined patient cohort. These features can be further appreciated in Figure 3, which compares multi-slice results obtained by both methods.Conclusion
While the number of patients in this study is small, data indicates that SPEN can provide images with at least the same diagnostic value as RESOLVE, underlying its potential to overcome geometric distortions for diagnosing cholesteatomas by DWI.Acknowledgements
Support from the Minerva Foundation (Germany), the Israel Science Foundation, the Clore Institute for Magnetic Resonance (Weizmann Institute), are acknowledged, as is the generosity of the Perlman Family and the Azrieli Foundations.References
(1) Semaan, M. T.; Megerian, C. A. Otolaryngol. Clin. North Am. 2006, 39, 1143.
(2) Persaud, R.; Hajioff, D.; Trinidade, A.; Khemani, S.; Bhattacharyya, M. N.; Papadimitriou, N.; Kalan, A.; Bhattacharyya, A. K. J. Laryngol. Otol. 2007, 121, 1013.
(3) Dremmen, M. H. G.; Hofman, P. A. M.; Hof, J. R.; Stokroos, R. J.; Postma, A. A. AJNR Am J Neuroradiol 2012, 33, 439.
(4) Li, P. M. M. C.; Linos, E.; Gurgel, R. K.; Fischbein, N. J.; Blevins, N. H. Laryngoscope 2013, 123, 1247.
(5) Muzaffar, J.; Metcalfe, C.; Colley, S.; Coulson, C. Clin. Otolaryngol. 2017, 42, 536.
(6) Algin, O.; Aydın, H.; Ozmen, E.; Ocakoglu, G.; Bercin, S.; Porter, D. A.; Kutluhan, A. J. Neuroradiol. 2017, 44, 388.
(7) Azuma, T.; Kodama, T.; Yano, T.; Enzaki, M.; Nakamura, M.; Murata, K. Magn. Reson. Med. Sci. 2015, 14, 145.
(8) Sheng, Y.; Hong, R.; Sha, Y.; Zhang, Z.; Zhou, K.; Fu, C. BMC Med. Imaging 2020, 20, 40.
(9) Cousin, S. F.; Liberman, G.; Solomon, E.; Otikovs, M.; Frydman, L. Magn. Reson. Med. 2019, 82, 1322.
(10) Schmidt, R.; Frydman, L. Magn. Reson. Med. 2014, 71, 711.
(11) Hansen, M. S.; Sørensen, T. S. Magn. Reson. Med. 2013, 69, 1768.
(12) Xue, H.; Inati, S.; Sørensen, T. S.; Kellman, P.; Hansen, M. S. Magn. Reson. Med. 2015, 73, 1015.
Figures