1773
Longitudinal resting-state functional connectivity changes with medication and nutrition treatment in chronic mild traumatic brain injury1Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
This study provides comprehensive evaluation of functional rs-fMRI biomarkers that are associated with chronic mTBI symptoms and predicting treatment outcome and patient recovery. We evaluate the effect of medication and nutrition treatments on brain function and cognitive performance in chronic mTBI patients using resting-state fMRI metrics and neuropsychological test. We propose that treatment strategies compensate mTBI disrupt communication between brain network nodes. Our results help understanding the implication of treatment strategies in chronic mTBI patients and prediction of outcomes in brain function and cognitive recovery.
Traumatic brain injury (TBI) is one of the most common neurological disorders accounting for more than 10’000’000 annual death and hospitalizations across the world 1,2. It is characterized by cognitive and emotional deficits within the first few weeks after injury that may persist by several months or years 3,4. A treatment choice between medication and nutrition therapy may be optimized combining with functional imaging biomarker in chronic mTBI patients. We assessed how these patients respond to these types of treatments. Further, cognitive performance recovery associated with the effect of the treatments has been estimated in this study.
Methods:
Resting-state functional magnetic resonance imaging (rs-fMRI) scans were acquired in 45 chronic mTBI patients at two time points: baseline (BL) and follow-up (FU) after 3 months of treatment randomized to nutrition (NUT) (n=12), N-acetyl cysteine (NAC) medication (n=20), controls (CN) (n=13). At each time point, voxel-wise whole brain rs-fMRI metrics including fractional amplitude of low frequency fluctuation (fALFF) and seed-base functional connectivity (FC) were measured for each patient using DPARSF_V5.0 after preprocessing steps5. Cognitive performance was assessed using Rivermead Post Concussion Symptoms Questionnaire (RPQ-13) and trail A, for each patient and at each time point. Two-sample t-test was conducted to obtain fALFF and seed-based FC differences between treatment groups (NAC and NUT) and CN. Partial correlation analysis was employed to estimate the association of rs-fMRI metrics with cognitive performance using age and gender as covariates. Brain networks including default mode network (DMN), executive control network (ECN), and dorsal attention network (DAS) were selected as the seeds for FC measurements and mask of ROIs for correlation analysis. In all the steps, BL measurements were subtracted from FU measurements (∆=FU-BL).
Results:
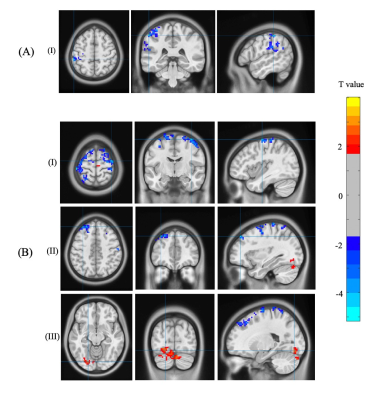
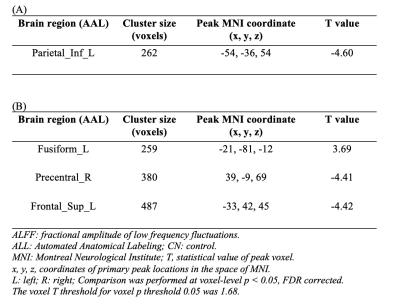
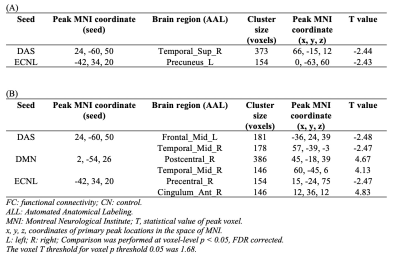
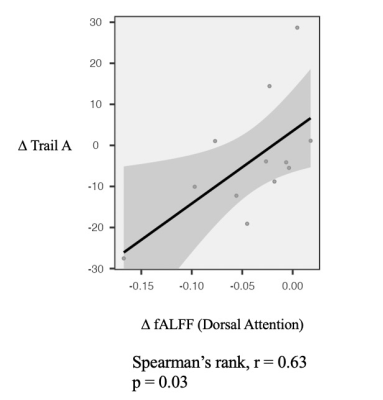
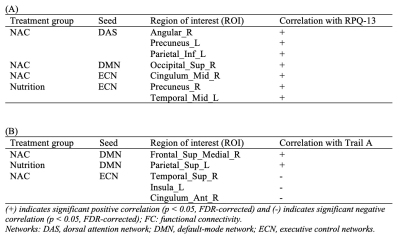
fALFF was significantly decreased in the Parietal_Inf_L in NAC compared to CN group. However, NUT group showed increased fALFF in the Fusiform_L, and decreased fALFF in Precentral_R and Frontal_Sup_L (Figure 1 and Table 1). Seed-based FC decreased between the seed located at DAS and Temporal_Sup_R in NAC group and decreased FC between DAS and Frontal_Mid_L Temporal_Mid_R in NUT group compared to CN. FC increased between seed located at DMN and Postcentral_R and Temporal_Mid_R in NUT group. Also, FC with seed located at the ECN decreased in Precuneus_L in NAC and decreased in Precentral_R and increased in Cingulum_Ant_R in NUT group compared to CN (Table 2). Regarding correlation analysis positive association has been found between trail A and fALFF in DAS in NUT group (Figure 2). Correlation analysis of seed-based FC showed significant positive correlation between RPQ-13 and alteration of FC between DAS and Angular_R, Precuneus_L, and Parietal_Inf_L, altered FC between DMN and Occipital_Sup_R, and altered FC between ECN and Cingulum_Mid_R in NAC group. Also, it showed positive correlation between RPQ-13 and alteration of FC between ECN and Precuneus_R and Temporal_Mid_L in the NUT group. Additionally, positive correlation between trail A and altered FC between DMN and Frontal_Sup_Medial_R in NAC group, positive correlation between trail A and altered FC between DMN and Parietal_Sup_L in NUT group, and negative correlation between trail A and altered FC between ECN and Temporal_Sup_R, Insula_L, and Cingulum_Ant_R have been shown in NAC group (Table 3).
Discussion:
This study provides comprehensive evaluation of functional rs-fMRI biomarkers that are associated with chronic mTBI symptoms and predicting treatment outcome and patient recovery. We characterized longitudinal changes in resting-state metrics as a function of recovery profile and estimated the effect of treatment with NAC medication and nutrition after 3 months. The results show that both fALFF and FC follow recovery profile in chronic mTBI6. Altered rs-fMRI metrics in the DMN after mTBI have been reported in the literature7,8. Additionally, a lot of studies have shown within-network and between-network FC alteration particularly in DMN, ECN, and DAS9–11. Our results are consistent with the literature showing that NAC and NUT treatments assist with cognitive recovery and adaptive response to cognitive impairment associated with chronic mTBI. Furthermore, we provide the evidence that cognitive performance recovery is more linked with between-network FC changes in this cohort.
Conclusion:
Our findings demonstrate novel rs-fMRI biomarker for chronic mTBI clinical outcome prediction. In addition, our results help understanding the implication of treatment strategies in chronic mTBI patients and prediction of outcomes in brain function and cognitive recovery. These results are very encouraging and warrant further investigation with higher sample size.
Acknowledgements
We thank the Marcus foundation at Thomas Jefferson University for support this study.References
1. Roozenbeek, B., Maas, A. I. R. & Menon, D. K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 (2013).
2. Sinke, M. R. T. et al. Imaging markers for the characterization of gray and white matter changes from acute to chronic stages after experimental traumatic brain injury. J. Neurotrauma 38, 1662–1669 (2021).
3. Mayer, A. R. et al. Static and Dynamic Intrinsic Connectivity following Mild Traumatic Brain Injury. J. Neurotrauma 32, 1046–1055 (2015).
4. Komura, A. et al. Cerebral Glucose Metabolism in Patients with Chronic Mental and Cognitive Sequelae after a Single Blunt Mild Traumatic Brain Injury without Visible Brain Lesions. J. Neurotrauma 36, 641–649 (2019).
5. Yan, C. G., Wang, X. Di, Zuo, X. N. & Zang, Y. F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351 (2016).
6. McCrea, M. et al. Immediate neurocognitive effects of concussion. Neurosurgery 50, 1032–1042 (2002).
7. Sharp, D. J. et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain 134, 2233–2247 (2011).
8. Madhavan, R. et al. Longitudinal Resting State Functional Connectivity Predicts Clinical Outcome in Mild Traumatic Brain Injury. J. Neurotrauma 36, 650–660 (2019).
9. Mayer, A. R., Bellgowan, P. S. F. & Hanlon, F. M. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev. 49, 8–18 (2015).
10. Dall’Acqua, P. et al. Functional and structural network recovery after mild traumatic brain injury: A 1-year longitudinal study. Front. Hum. Neurosci. 11, 1–16 (2017).
11. Zhou, Y. et al. Default-mode network disruption in mild traumatic brain injury. Radiology 265, 882–892 (2012).
Figures