1770
Hippocampal subfield neurofunctional alterations in chronic insomnia1Suining Central Hospital, Chuanshan Suining, China
Synopsis
This study investigated the patterns of volume changes across neurofunctionally distinct hippocampal subfields in patients with chronic insomnia. The results suggested that L1 and R2 atrophy increased the risk of developing chronic insomnia approximately 3-fold, and negative emotion positively acted on the causal path of insomnia severity leading to R1 atrophy. In addition, we developed a practical and visual nomogram of individual competing risks for predicting chronic insomnia risk with high accurate predictive power based on these hippocampal subfields. Neurofunctional hippocampal subfield atrophy was a risk factor leading to chronic insomnia, contributing to understand of the etiology of insomnia.
INTRODUCTION
Chronic insomnia is one of the most prevalent health complaints worldwide1. Chronic insomnia increases the risk of dementia and is associated with mood disturbances and cognitive function deficits2-5. The hippocampus plays a crucial role in the acquisition, consolidation, and recovery of memory6,7. Different hippocampal subfields are responsible for different cognitive functions and are affected by neuropsychiatric conditions to varying degrees8. Despite a recent increase in studies concerning the association of hippocampal volume (HV) with chronic insomnia, consistent findings have not been obtained. These studies evaluated structural hippocampal atrophy; however, to our knowledge, no study has performed a comprehensive examination to identify and characterize neurofunctional subfields of HV in chronic insomnia. The aim of the current study was to investigate the pattern of volume changes across neurofunctionally distinct hippocampal subfields in patients with chronic insomnia compared to GSs.METHODS
A total of 120 patients with chronic insomnia (78 females, 42 males; mean age ± std, 43.74 ± 13.02 years) and 120 good sleepers (60 females, 60 males; mean age, 42.69 ± 12.24 years) were recruited (table 1). All subjects were asked to complete a number of questionnaires, including the Pittsburgh Sleep Quality Index (PSQI), Self-Rating Anxiety Scale (SAS), and Self-Rating Depression Scale (SDS). MRI scans were performed on 3-Tesla MR scanners (Trio, Siemens, Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired with a three-dimensional spoiled gradient-recalled sequence in a sagittal orientation: 176 images (repetition time = 1900 ms, echo time = 2.26 ms, thickness = 1.0 mm, gap = 0.5 mm, acquisition matrix = 256 × 256, field of view = 250 mm × 250 mm, flip angle = 9°0) were obtained. The left hippocampus was segmented into anterior (L1), middle (L2), and posterior (L3) subregions. The right hippocampus was segmented into top anterior (R1), second top anterior (R2), middle (R3), posterior (R4), and last posterior (R5) subregions. Multivariate logistic regression was used to evaluate hippocampal subfield associations with the risk of chronic insomnia. Mediation analyses were performed to evaluate mediated associations with postinsomnia negative emotion, insomnia severity and hippocampal atrophy. A visual easy-to-deploy risk nomogram was used for individual predictions of insomnia risk.RESULTS
Hippocampal atrophy was identified in the L1, R1, and R2 after controlling for total intracranial volume (TIV)(table 2). L1 and R2 atrophy each predisposed patients to an approximate 3-fold higher risk of chronic insomnia (L1, OR: 2.90 [95% CI: 1.24, 6.76], P = 0.014; R2, (2.72 [1.19, 6.20]), p = 0.018). We developed a practical and visual competing risk nomogram for individual predictions of insomnia risk based on these hippocampal subfields, which stratified individuals into different levels of insomnia risk with the highest prediction accuracy of 97.4% and average C-statistic of 0.83 (figure 1). Anxiety fully mediated the causal path of insomnia severity leading to R1 volume atrophy (table 3 and figure 2).DISCUSSION
In this case-control study with a relatively large sample size, four main novel findings are worth noting. First, hippocampal atrophy was identified in three subfields, including the L1, R1, and R2, in these patients with chronic insomnia after controlling for TIV. These neurofunctional subfields are mainly associated with negative emotions. Second, L1 and R2 atrophy each predisposed individuals to an approximate 3-fold higher risk of chronic insomnia. Third, PSQI scores were negatively correlated with R1 volume, and anxiety was a positive mediator in the causal path of insomnia severity leading to R1 volume atrophy. These findings suggest that chronic insomnia is associated with emotional and cognitive processing disorders and highlight the role of negative emotion and corresponding hippocampal neurofunctional subfields in the aetiology of chronic insomnia.Fourth, we developed a practical and visual competing risk nomogram for individual predictions of risk for developing chronic insomnia based on atrophy in hippocampal subfields, which stratified individuals into different levels of insomnia risk with the highest prediction accuracy of 97.4% and average C-statistic of 0.83.CONCLUSION
Chronic insomnia was associated with neurofunctional hippocampal subfield atrophy, which was a risk factor for chronic insomnia. These findings could broaden our understanding of the aetiology of insomnia, help individuals identify their potential risk profile and provide guidance for individuals to take precise actions in a timely manner to prevent the future incidence of insomnia.Acknowledgements
No acknowledgement found.References
- 1. Morin CM, Benca R. Chronic insomnia. Lancet (London, England). 2012; 379 (9821): 1129-1141. 2. Sexton CE, Sykara K, Karageorgiou E, et al. Connections Between Insomnia and Cognitive Aging. Neuroscience bulletin. 2020; 36 (1): 77-84.
- 3. Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014; 10: 2163-2175.
- 4. Dai XJ, Liu BX, Ai S, et al. Altered inter-hemispheric communication of default-mode and visual networks underlie etiology of primary insomnia : Altered inter-hemispheric communication underlie etiology of insomnia. Brain Imaging Behav. 2020; 14 (5): 1430-1444.
- 5. Nie X, Shao Y, Liu SY, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treat. 2015; 11: 3085-3093.
- 6. Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature reviews Neuroscience. 2000; 1 (1): 41-50.
- 7. Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences. 2000; 911: 1-24.
- 8. Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011; 21 (9): 923-928.
Figures
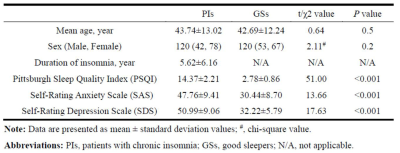
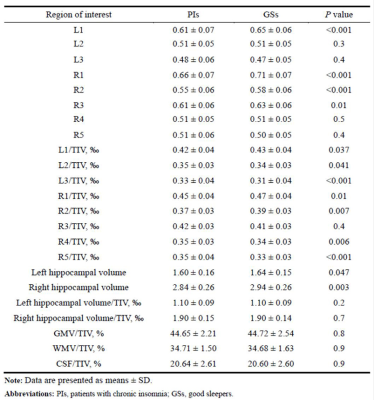
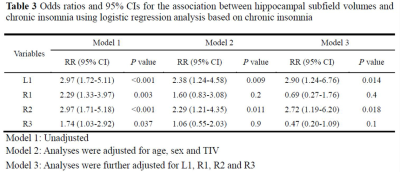
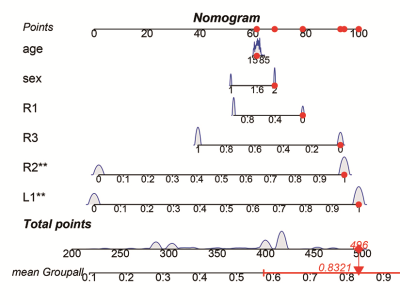
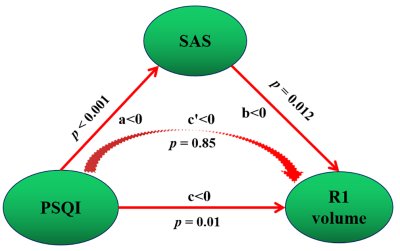
Figure 2 Mediation association of postinsomnia negative emotion, insomnia severity and hippocampal atrophy
Note: PSQI scores were negatively correlated with R1 volume, which indicates a positive correlation with R1 volume atrophy. SAS scores were negative acting in the causal path of PSQI leading to R1 volume; that is, SAS scores were positive acting in the causal path of PSQI leading to R1 volume atrophy.