1769
Increased GABA in the periaqueductal grey of patients with chronic low back pain detected using 1H-magnetic resonance spectroscopy1Department of Chiropractic Medicine, Integrative Spinal Research Group, Balgrist University Hospital, University of Zurich, Zurich, Switzerland, 2University of Zurich, Zurich, Switzerland, 3Neuroscience Center Zurich, University of Zurich, Zurich, Switzerland, 4Department of Forensic Medicine and Imaging, Institute of Forensic Medicine, University of Zurich, Zurich, Switzerland, 5Department of Psychiatry, Hospital of Psychiatry, Psychotherapy and Psychosomatics, University of Zurich, Zurich, Switzerland
Synopsis
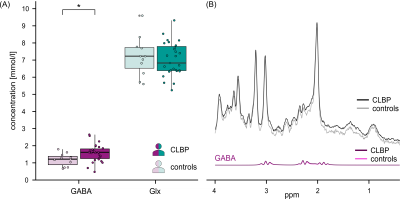
Preclinical studies suggest a role of altered γ-aminobutyric acid (GABA)ergic inhibitory tone in the periaqueductal grey (PAG), a key brain area for descending pain modulation, as mechanism contributing to chronic pain. The present 1H-magnetic resonance spectroscopy study investigated GABA, and glutamate/glutamine, concentrations in the PAG of patients with chronic low back pain (CLBP). Using a point resolved spectroscopy sequence with optimized frequency alignment, adequate spectral quality was achieved and increased GABA concentrations (p=0.027) identified in CLBP patients compared to pain-free controls. This finding supports dysregulations in descending pain modulation as factor contributing to chronic pain in humans.
INTRODUCTION
Mechanisms underlying chronic pain are insufficiently understood, resulting in a lack of targeted and satisfactory treatment options. Preclinical evidence points towards a potential contribution of maladaptive plasticity in pain-relevant brain areas, such as imbalances of excitatory glutamatergic and inhibitory γ-aminobutyric acid (GABA)ergic neurotransmission1. The balance between excitation and inhibition is particularly important in a key region of descending pain modulation, i.e. the periaqueductal grey (PAG)2, which exerts antinociceptive effects upon excitation and pronociceptive effects upon inhibition3. In a preclinical model of neuropathic pain, increased postsynaptic inhibitory currents were observed in the PAG4, suggesting enhanced presynaptic GABA release. Whether and how such changes occur in the human PAG remains unknown. In this study, we used a point resolved spectroscopy (PRESS) sequence with optimized frequency alignment5,6 to measure concentrations of GABA and glutamate/glutamine (as complementary measure of excitatory tone) in the PAG of patients with chronic low back pain (CLBP), a prevalent chronic pain condition7. It was expected that the GABAergic tone would be increased in patients with CLBP compared to pain-free controls.METHODS
Data acquisition: Sixty CLBP patients and 30 age- and sex-matched pain-free controls undergo a magnetic resonance imaging session on a 3T system (Philips Achieva, 32-channel head coil) including acquisition of: (1) 3D T1-weighted images (TE/TR/TI=3.7/8.1/1024 ms, shot interval=3000ms, voxel (size)=1mm³ isotropic, FOV (AP/RL/FH)=240/160/240mm³) and (2) 1H-MRS in the periaqueductal grey (PRESS, TE/TR=33/2500ms, NSA=64, voxel=11/15/18mm =3mL, VAPOR water suppression8). Voxels were placed according to anatomical landmarks.Data analysis: 1H-MRS data were exported and processed using frequency alignment, phase correction, and eddy current correction6. Peak areas of the different metabolites were fitted using LCModel and simulated basis sets6,9. The main metabolites of interest were GABA and glutamate/glutamine (Glx). Relative volume fractions of brain compartments in the measured spectroscopic voxels were determined using the T1-weighted 3D TFE data sets. The images were segmented into cerebrospinal fluid, grey matter and white matter compartments using SPM1210.
Statistical analysis: GABA and Glx concentration estimates (in mmol/l) were compared between CLBP patients and controls using a Wilcoxon rank sum test11. Statistical analyses were performed in R 4.1.0/RStudio 1.4.1717 for Mac.
RESULTS
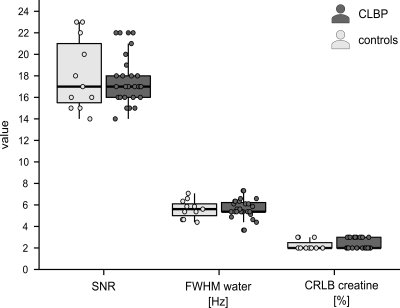
Of the 30 CLBP patients and 11 controls measured to date, data from 3 CLBP patients were excluded due to insufficient quality (motion artifacts, irregular noise patterns). In the remaining 27 CLBP patients (14 F, age mean±SD=38.0±18.7y) and 11 controls (7 F, age mean±SD=49.7±17.4y) spectra presented with consistent high signal-to-noise ratios, narrow line widths of the water peak and stable Cramér-Rao lower bounds for creatine (Figure 1). GABA was significantly increased (+32.7%) in CLBP patients compared to controls (W=76, p=0.027), while Glx concentration estimates did not significantly differ between the cohorts (W=154, p=0.87) (Figure 2).DISCUSSION
The PAG plays a crucial role in endogenous pain inhibition. One of its main antinociceptive pathways involves activation of glutamatergic PAG projections to the rostral ventromedial medulla where descending inhibition of spinal dorsal horn neurons is initiated2. The glutamatergic PAG projections themselves are under tonic GABAergic control. In this context, it is conceivable that an increased GABAergic tone in the PAG, as detected in the present study, impedes the activation of this antinociceptive pathway, possibly contributing to enhanced pain sensitivity in chronic pain states.Performing MRS in the human PAG is challenging due to several factors: constant cerebrospinal fluid flow through the cerebral aqueduct, movement with heart pulsation and breathing, as well as its small size. Accurate and specific metabolite detection becomes particularly challenging for metabolites with low concentrations, such as GABA. Nevertheless, the quality of the herein recorded spectra is adequate, with water linewidths (5.7±0.8Hz) considered excellent12 and signal-to-noise ratios (mean 17.7) similar to a study which used a comparable voxel size in the brainstem (mean 19.1)13. A limitation of PRESS sequences remains the uncertainty about the specificity of the detected GABA signals, i.e., the GABA signal might partly be attributable to metabolites with similar frequency spectra. Other methods, such as MEGA-PRESS14,15 would be advantageous in this regard. However, the use of editing pulses is not suited for such unstable environments as in the PAG nor for small voxel sizes16. We propose that PRESS with optimized frequency alignment5,6 is an adequate method to detect metabolite concentrations in the PAG, provided that uncertainties regarding GABA specificity are acknowledged. Confirmation of results by other fitting tools, e.g. OSPREY17, might provide further support of the findings and is envisioned for the analysis of the complete dataset.
CONCLUSION
Chronic pain conditions belong to the most burdensome disorders worldwide7 and improving the understanding of underlying mechanism is crucial to advance available treatment options. The present study detected increased GABA concentrations in the PAG of CLBP patients compared to pain-free controls. Expanding the methodology to other chronic pain disorders might allow insights into the role of altered descending pain modulation across disorders.Acknowledgements
This study is funded by the Clinical Research Priority Program University of Zurich CRPP “Pain”. The authors express their gratitude to Emma Louise Kessler, MD for her generous donation to the Zurich Institute of Forensic Medicine, University of Zurich, Switzerland.
References
1 Kuner, R. & Kuner, T. Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol Rev. 2021; 101: 213-258.
2 Basbaum, A. I. & Fields, H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984; 7: 309-338.
3 Samineni, V. K., Grajales-Reyes, J. G., Copits, B. A. et al. Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray. eNeuro. 2017; 4.
4 Hahm, E. T., Kim, Y., Lee, J. J. et al. GABAergic synaptic response and its opioidergic modulation in periaqueductal gray neurons of rats with neuropathic pain. BMC Neurosci. 2011; 12: 41.
5 Near, J., Edden, R., Evans, C. J. et al. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015; 73: 44-50.
6 Simpson, R., Devenyi, G. A., Jezzard, P. et al. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017; 77: 23-33.
7 Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390: 1211-1259.
8 Tkac, I., Starcuk, Z., Choi, I. Y. et al. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999; 41: 649-656.
9 Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993; 30: 672-679.
10 Ashburner, J., Barnes, G., Chen, C. et al. SPM12 Manual. Functional Imaging Laboratory, Institute of Neurology. 2021.
11 Wilcoxon, F. Individual Comparisons by Ranking Methods. Biometrics Bulletin. 1945; 1: 80-83.
12 Oz, G., Deelchand, D. K., Wijnen, J. P. et al. Advanced single voxel (1) H magnetic resonance spectroscopy techniques in humans: Experts' consensus recommendations. NMR Biomed. 2020: e4236.
13 de Matos, N. M. P., Hock, A., Wyss, M. et al. Neurochemical dynamics of acute orofacial pain in the human trigeminal brainstem nuclear complex. Neuroimage. 2017; 162: 162-172.
14 Mescher, M., Tannus, A., Johnson, M. O. n. et al. Solvent Suppression Using Selective Echo Dephasing. Journal of Magnetic Resonance, Series A. 1996; 123: 226-229.
15 Mescher, M., Merkle, H., Kirsch, J. et al. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998; 11: 266-272.
16 Mullins, P. G., McGonigle, D. J., O'Gorman, R. L. et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014; 86: 43-52.
17 Georgiev, I., Roberts, K. E., Gainza, P. et al. OSPREY (Open Source Protein Redesign for You) user manual. 2009.
Figures