1764
Prospective Evaluation of Machine Learning Prescription of Cardiac MRI Planes in Children with Congenital Heart Disease1Department of Radiology, Stanford University, Palo Alto, CA, United States, 2GE Healthcare, Menlo Park, CA, United States
Synopsis
Cardiac MRI plays an important role in defining cardiac anatomy and function in children with heart disease. Prescription of diagnostic planes in cardiac MRI requires specialized training, limiting availability. Machine-learning based prescription has the potential to allow rapid, consistent acquisition of these planes independent of operator, but performance in congenital heart disease is unknown. This study evaluates the utility of such a system which demonstrates performance in children with mild to moderate heart disease similar to healthy adult volunteers. As expected, the system does not generate acceptable results in severe intracardiac anomalies.
Introduction
Cardiac MRI is vital for diagnostic evaluation, pre-surgical planning, and longitudinal follow-up of children with congenital or genetic cardiac anomalies [1–4]. It requires the sequential prescription of specific imaging planes by a physician or a specialized technologist [5], which may limit widespread adoption [6]. Machine learning (ML) can automate the prescription of cardiac scan planes [7]. Nevertheless, the robustness of such models in the presence of abnormal anatomy is unknown. As congenital heart disease (CHD) affects up to 1 in 200 live births and may be undiagnosed [8], robustness to various forms of congenital or genetic heart disease is desired. Our study assesses the real-world performance of such a system within a clinical workflow at a tertiary care pediatric hospital.Methods
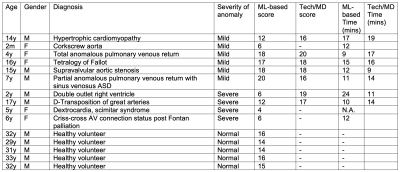
With institutional review board approval, 10 pediatric patients who underwent 3T cardiac MRI (Discovery 750, GE Healthcare) for various clinical indications and 5 adult volunteers from October 2021 to November 2021 were included. Most patients had known or suspected CHD. Balanced steady state free precession (SSFP) images were acquired in short axis (SAX), 4-chamber (4CH), left ventricular 3-chamber (LV3CH), and left ventricular 2-chamber (LV2CH) planes. These planes were automatically prescribed by an investigational machine-learning algorithm running real-time on the scanner. The algorithm uses neural networks trained on mostly adults with normal anatomy and some patients with mild congenital heart disease (CHD) to identify anatomic landmarks [7], which then determine the planes. The field-of-view was adjusted to accommodate different subject sizes, but planes as prescribed were acquired without modification. In the patients, diagnostic planes were also acquired by a specialized technologist with >15 years of experience. Such paired data was acquired in 7 patients; clinical status in the remaining 3 patients precluded additional imaging.Each of the planes was graded qualitatively by a cardiac imager with expertise in CHD. Scores between 1-5 corresponded to very poor, poor, acceptable, good, and excellent planes respectively. The scores from each plane were summed for a composite score. Research subjects were separated into three groups: volunteers (n=5), patients with mild-moderate CHD (n=6), and patients with severe CHD (n=4). Scores between the volunteers vs. mild CHD, and volunteers vs. severe CHD were compared using two-tailed t-tests. The amount of time required to acquire each set of planes using ML-based prescription was computed from timestamps and summarized. Where paired data was acquired, manually prescribed planes were also scored and their acquisition time was also computed.
Results
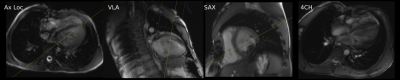
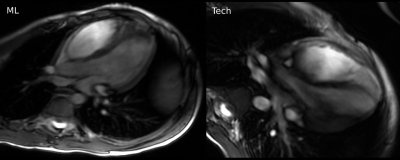
Pediatric patients included 5 males and 5 females (mean age 8.5 years). The volunteers included 5 males (mean age 31.4 years). All patients had congenital heart disease, except one patient with genetic heart disease (hypertrophic cardiomyopathy) (Figure 1).The ML-based prescription achieved mean scores of 15, 15.2, and 7 in volunteers, mild-moderate CHD patients, and severe CHD patients respectively. Scores from volunteers was significantly higher than in severe CHD patients (p=0.015). No significant difference was found between the scores of the volunteers and patients with mild-moderate CHD (p=0.942). The ML-based prescription demonstrated good performance in healthy volunteers (Figure 2) and in mild-moderate CHD (Figure 3), occasionally outperforming prescription by the technologist (Figure 4).
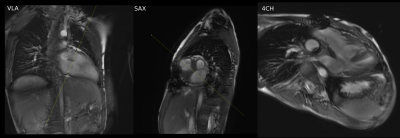
ML-based plane prescription was not adequate in severe CHD, e.g. criss-cross atrioventricular connection (Figure 5). The mean time required to prescribe and acquire the planes for ML-based prescription was 13.6 minutes vs. 14.3 minutes for manual prescription.
Paired data was available in 7 patients. The scores from manual prescription were higher than those acquired by ML-based prescription in patients with severe CHD but were similar in patients with mild to moderate CHD (Figure 1).
Discussion
Our pilot study demonstrates the feasibility of integrating ML-based prescription into the clinical workflow. Retrospectively assessed performance is excellent [7,9,10], and results in our volunteers were consistent with this. The ML-based prescriptions scored similarly and occasionally outperformed an experienced cardiac technologist in patients with mild-moderate CHD.The model’s ability to prescribe diagnostic planes in cases of CHD indicates the robustness and generalizability to images that may be outside the training data. However, our experience with severe CHD demonstrates limitations of this generalization. Prescription of planes for these patients is challenging even for experienced cardiac imagers and technologists. Such prescription would be difficult without training specifically for these abnormalities. These diagnoses are unlikely to be seen outside of specialized centers. It is important to highlight the current limitations of ML-based prescription, and further training is needed to develop networks that are capable of prescription in these edge cases.
Automated prescription allowed the acquisition of a set of cardiac planes in a mean time of 13.6 minutes and has the potential to increase efficiency, although in our study, the time for ML-based prescription was similar to manual prescription. Further study to determine any true improvements in efficiency are warranted.
The main limitation of our pilot study is the small sample size, so our results are exploratory and require validation in larger prospective cohorts.
Conclusion
Automated ML-based plane prescription for cardiac MRI is clinically feasible and allows efficient acquisition of cardiac MRI. Planes prescribed in normal volunteers and in mild-moderate CHD are acceptable. Additional work is needed to produce acceptable planes in patients with severe CHD.Acknowledgements
No acknowledgement found.References
1. Seraphim A, Knott KD, Augusto J et al. (2020) Quantitative cardiac MRI. J Magn Reson Imaging John Wiley & Sons, Ltd. 51:693–711. https://doi.org/10.1002/JMRI.26789
2. Fratz S, Chung T, Greil GF et al. (2013) Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013 151 BioMed Central. 15:1–26. https://doi.org/10.1186/1532-429X-15-51
3. Ntsinjana HN, Hughes ML and Taylor AM (2011) The Role of Cardiovascular Magnetic Resonance in Pediatric Congenital Heart Disease. J Cardiovasc Magn Reson 2011 131 BioMed Central. 13:1–20. https://doi.org/10.1186/1532-429X-13-51
4. Kellenberger CJ, Yoo S-J and Büchel ERV (2007) Cardiovascular MR Imaging in Neonates and Infants with Congenital Heart Disease. Https://DoiOrg/101148/Rg271065027 Radiological Society of North America. 27:5–18. https://doi.org/10.1148/RG.271065027
5. Suinesiaputra A, Bluemke DA, Cowan BR et al. (2015) Quantification of LV function and mass by cardiovascular magnetic resonance: multi-center variability and consensus contours. J Cardiovasc Magn Reson 2015 171 BioMed Central. 17:1–8. https://doi.org/10.1186/S12968-015-0170-9
6. Goldfarb JW and Weber J (2021) Trends in Cardiovascular MRI and CT in the U.S. Medicare Population from 2012 to 2017. Https://DoiOrg/101148/Ryct2021200112 Radiological Society of North America. 3. https://doi.org/10.1148/RYCT.2021200112
7. Blansit K, Retson T, Masutani E, Bahrami N and Hsiao A (2019) Deep Learning-based Prescription of Cardiac MRI Planes. Radiol Artif Intell Radiol Artif Intell. 1:e180069. https://doi.org/10.1148/RYAI.2019180069
8. Hoffman JIE and Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol J Am Coll Cardiol. 39:1890–900. https://doi.org/10.1016/S0735-1097(02)01886-7
9. Frick M, Paetsch I, Den Harder C et al. (2011) Fully automatic geometry planning for cardiac MR imaging and reproducibility of functional cardiac parameters. J Magn Reson Imaging 34:457–67. https://doi.org/10.1002/JMRI.22626
10. X L, MP J, B G et al. (2011) Automatic view planning for cardiac MRI acquisition. Med Image Comput Comput Assist Interv Med Image Comput Comput Assist Interv. 14:479–86. https://doi.org/10.1007/978-3-642-23626-6_59
Figures