1758
Accelerated Multiband Cine Cardiac Magnetic Resonance Imaging using Deep Learning1United Imaging Intelligence, Cambridge, MA, United States, 2UIH America, Inc., Houston, TX, United States
Synopsis
Cine CMR is a routinely performed technique for heart anatomy and function analysis. In clinics, repeated breathholdings are performed to acquire multiple 2D slices to cover the whole heart, which further exacerbates the discomfort and challenges for young and severe-diseased patients. In this study, we halved the breathholding time for cine CMR using MB technique. Multiple sampling patterns were designed and studied for the best reconstruction quality. A NN leveraging the Siamese structure was designed to reconstruct the accelerated MB data and achieved great image quality.
INTRODUCTION
Cine Cardiac Magnetic Resonance (CMR) imaging is a routinely performed technique for heart anatomy and function analysis. On the cine images, a complete contraction-relaxation cycle of cardiac motion is recorded, which requires breathholding to freeze the respiratory motion. In clinics, repeated breathholdings are performed to acquire multiple 2D slices to cover the whole heart, which further exacerbates the discomfort and challenges for young and severe-diseased patients. Through the years, tremendous efforts have been made to accelerate cine imaging by undersampling data for each slice1-8, where high acceleration rates have been achieved to acquire a single slice cine in one heartbeat; however, further in-slice acceleration cannot alleviate the breathholding issue. Autocalibrated multiband (MB) or simultaneous multi slice (SMS) imaging9,10 where multiple slices are excited at the same time and uses reconstruction to disentangle the mixed slice signal can potentially address this issue. Some studies explored compressed sensing11 but the reconstruction time is long for practical use. Recently deep learning (DL) based reconstruction methods have shown promising results for many MR applications4-8,12. However, the work in [12] uses convolutional neural network (NN) in the kspace domain and exploits coil correlations for brain imaging, which may not be suitable for CMR since the coil configuration is usually poor along the slice direction. In this study, we propose a specially designed neural network (NN) to accelerate MB cine CMR, which was validated both prospectively and retrospectively. To our best knowledge, this is the first DL accelerated MB cine CMR work.METHODS
The MB cine sequence was based on the auto-calibrated MB CAIPIRINHA9. Phase modulation is achieved by adjusting the RF excitation phase $$$\theta_{i}$$$ for the $$$i^{th}$$$ slice. The collected phase-encoding (PE) data is $$$S^{MB}(k_{y})=\sum_{i}S^{i}(k_{y})\cdot e^{-j\theta_{i}}$$$, where $$$\theta_{i}\in\{0,\frac{2\pi}{M},..., \frac{2\pi}{M}(M-1)\}$$$ for a given MB factor $$$M$$$ (number of SMS slices). A single-band bSSFP cine sequence was modified using the multiband RF pulses for the prospective MB acquisition. Retrospective MB acquisition was also implemented to synthesize MB data from multi-slice SB cine. A Cartesian trajectory was followed and both in-slice and through-slice (MB) accelerations were used.A neural network is designed to reconstruct the accelerated MB cine data (Fig.1). Because SMS slices are not continuous in space, convolution along the slice direction cannot be applied to learn features. Instead, we used a Siamese NN structure to exploit the correlations among the SMS slices. The slices are fed to their corresponding sub-networks that shared the same weights. The sub-network, which only sees single slice cine images, is a convolutional recurrent neural network (CRNN) with a bi-directional data flow to model dynamic information, a data flow across iterations and residual connections for high-spatial-frequency information8. In the data consistency layer, by exploiting the Fourier property, the MB kspace data can be converted to a 3D kspace data and use 3D FFT pairs to transform between image and kspace. The new image estimations are then fed to the CRNN for the next iteration and total 5 iterations were used. No coil sensitivity maps were needed and the correlation among coils were inherently learned through training. During training, the NN output was compared to ground truth images to calculate MSE and SSIM for loss.
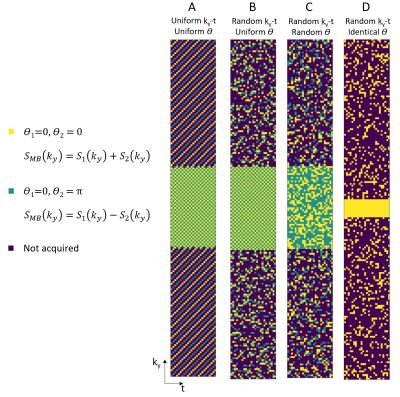
About 1.7K fully-sampled single-slice cine kspace data was used to generate about 9.3K retrospective MB cine data with multiple augmentation methods. Through-slice acceleration factor was 2 ($$$M=2$$$) and in-slice acceleration factor was 2, giving a total acceleration rate of 4. Four types of sampling strategies were studied by introducing randomness along different dimension(s) (Fig.2). A subset of the data was used to test different sampling patterns and for each pattern a separate NN was trained. The best sampling pattern was then used to generate all the data. The prospective MB cine was implemented using the best sampling pattern and data was collected from 10 volunteers approved by local IRB on a 3.0T scanner (United Imaging Healthcare, Shanghai, China). Other parameters include: TR 3.3ms, TE 1.7ms, FA 40, resolution 2.2x1.9mm, scan time 10 heartbeat. Single-slice cine was also collected for each volunteer as reference.
RESULTS
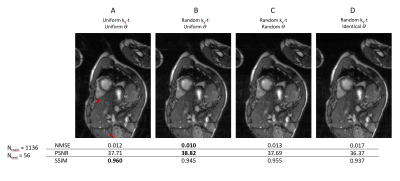
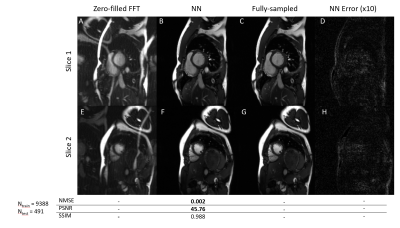
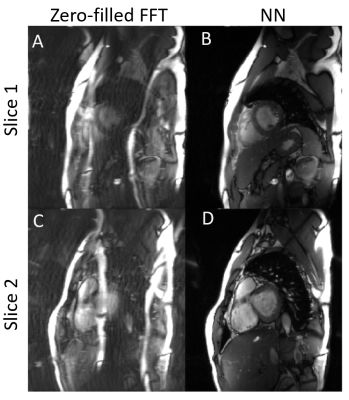
Four sampling patterns are compared in Fig.3. Some residual artifacts can be observed on uniform in-slice undersampling but not on others with randomness. Quantitative results show the overall best sampling pattern to be random $$$k_y-t$$$+ uniform $$$\theta$$$. Example reconstructions and quantitative results using the best sampling pattern are shown in Fig.4. The DL results closely resemble the ground truth images. In the prospective study (Fig.5), residual artifacts due to undersampling were removed although some flow artifacts can be observed. The average reconstruction time for one MB cine data of NN was less than 1 sec.DISCUSSIONS AND CONCLUSIONS
In this study, we halved the breathholding time for cine CMR using MB technique. Multiple sampling patterns were designed and studied for the best reconstruction quality. A NN leveraging the Siamese structure was designed to reconstruct the accelerated MB data and achieved great image quality. The fast reconstruction time of NN allows future clinical translation. Future studies are guaranteed for more NN ablations and perform cardiac function analysis on the MB cine data.Acknowledgements
No acknowledgement found.References
1. Tsao J, Boesiger P, Pruessmann KP (2003) k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med 50(5):1031–1042. https://doi.org/10.1002/mrm.10611
2. Lingala S, Hu Y, Dibella E, Jacob M (2011) Accelerated dynamic MRI exploiting sparsity and low-rank structure: K-t SLR. IEEE Trans Med Imaging 30(5):1042–1054. https://doi.org/10.1109/TMI.2010.2100850
3. Vincenti G, Monney P, Chaptinel J et al (2014) Compressed sensing single–breath-hold CMR for fast quantification of LV function, volumes, and mass. JACC Cardiovasc Imaging 7(9):882–892. https://doi.org/10.1016/j.jcmg.2014.04.016
4. J. Schlemper, J. Caballero, J. V. Hajnal, A. N. Price and D. Rueckert, "A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction," in IEEE Transactions on Medical Imaging, vol. 37, no. 2, pp. 491-503, Feb. 2018, doi: 10.1109/TMI.2017.2760978.
5. Hauptmann A, Arridge S, Lucka F, Muthurangu V, Steeden JA. Real-time cardiovascular MR with spatio-temporal artifact suppression using deep learning-proof of concept in congenital heart disease. Magn Reson Med. 2019 Feb;81(2):1143-1156. doi: 10.1002/mrm.27480. Epub 2018 Sep 8. PMID: 30194880; PMCID: PMC6492123.
6. K.H. Jin, H. Gupta, J. Yerly, M. Stuber, and M. Unser. Time-dependent deep image prior for dynamic MRI. arXiv:1910.01684, 2019.
7. C. Qin, J. Schlemper, J. Caballero, A. N. Price, J. V. Hajnal and D. Rueckert, "Convolutional Recurrent Neural Networks for Dynamic MR Image Reconstruction," in IEEE Transactions on Medical Imaging, vol. 38, no. 1, pp. 280-290, Jan. 2019, doi: 10.1109/TMI.2018.2863670.
8. E. Chen, X. Chen, J. Lv, Y. Zheng, T. Chen, J. Xu, and S. Sun. Real-Time Cardiac Cine MRI with Residual Convolutional Recurrent Neural Network. ISMRM 2020:3588
9. Ferrazzi G, Bassenge JP, Wink C, Ruh A, Markl M, Moeller S, Metzger GJ, Ittermann B, Schmitter S. Autocalibrated multiband CAIPIRINHA with through-time encoding: Proof of principle and application to cardiac tissue phase mapping. Magn Reson Med. 2019 Feb;81(2):1016-1030. doi: 10.1002/mrm.27460. Epub 2018 Sep 17. PMID: 30295955.
10. Price AN, Cordero-Grande L, Malik SJ, Hajnal JV. Simultaneous multislice imaging of the heart using multiband balanced SSFP with blipped-CAIPI. Magn Reson Med. 2020;83(6):2185-2196. doi:10.1002/mrm.28086
11. Zheng Y, Zhao L, Zhang Z, Ding Y, Xu J. Auto-calibrated simultaneous multiband cardiac GRE cine MRI at 5 Tesla. ISMRM 2021:4188
12. Zhang C, Moeller S, Weingärtner S, Uğurbil K, Akçakaya M. Accelerated Simultaneous Multi-Slice MRI using Subject-Specific Convolutional Neural Networks. Conf Rec Asilomar Conf Signals Syst Comput. 2018;2018:1636-1640. doi:10.1109/ACSSC.2018.8645313
Figures