1757
Whole-Heart Free-Breathing 3D Cardiac Cine with Fast Respiratory Motion Compensated Reconstruction1Philips Research, Hamburg, Germany
Synopsis
Whole-Heart, Free-Breathing 3D Cardiac Cine MRI with a cartesian, water-selective, balanced SSFP acquisition and spiral-like undersampling is combined with fast, non-rigid motion-compensated reconstruction. It demonstrates the feasibility of routine cardiac cine MRI with minimal planning effort and minimum patient discomfort by avoiding the need for breathholds. The combined acquisition and reconstruction time is below 5 minutes, including computation of deformation vector fields, and minimizes image production delay by starting reconstruction on partially acquired data.
Introduction
Widespread routine adoption of cardiac MRI is hampered by the complicated workflow associated with planning the correct scan orientations. In addition, the examination is also burdensome for patients who must hold their breath to freeze respiratory motion. This is particularly relevant for cardiac MRI where patients are likely to be short of breath. Whole-Heart Free-Breathing 3D Cardiac Cine Imaging overcomes the need for intricate planning, enables retrospective multi-planar reformatting in any desired orientation, and reduces patient discomfort [1-3]. Despite these advantages, Whole-Heart Free-Breathing 3D Cardiac Cine is challenging due to reduced contrast (reduced inflow effect), long acquisition times, even longer reconstruction times, and bears the risk of severe image degradation due to bright fat signal. Here we combine a free-running, water-selective, cartesian balanced SSFP acquisition with spiral-like undersampling (CASPR) on one hand, and and a fast, non-rigid motion-compensated reconstruction on the other hand, enabling clinically feasible acquisition and reconstruction times with minimal delay from the end of the scan to the reconstructed images.Methods
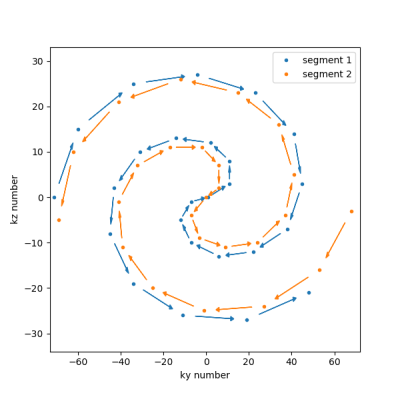
A free-running balanced SSFP sequence was modified to acquire segments of k-space ($$$k_y$$$, $$$k_z$$$) arranged as concatenation of an inward and outward spiral, see Figure 2, with the center of k-space acquired in each segment for respiratory self-navigation, similar to Refs. [4, 1]. 50 profiles were acquired in each segment. The ECG signal was recorded for retrospective binning into cardiac phases. Abbreviated binomial (1-1) water-selective pulses [5] were employed to excite a coronal slab covering the whole heart of 5 male volunteers (age 35-55 years, with informed consent) using a 28-channel anterior/posterior array coil on a 1.5T system (Ingenia, Philips, Best, The Netherlands) with modified software. Further scan parameters were TR/TE: 3.9/1.9 ms, 2.5 mm isotropic resolution, FOV (AP/RL/FH): (120/360/240) mm, Readout: FH, BW: 1080 Hz/voxel, FA: 65°. The total acquisition time was 6-7 minutes of which only the initial part was used, as described below.Reconstruction was performed in a series of steps, see also schematic in Figure 1. All computations were performed offline using Python and Pytorch for GPU (RTX5000, Nvidia) acceleration.
1) Respiratory self-navigation was performed using the 1D projection in the feet-head direction obtained from the k=0 profiles measured in each shot/segment. Singular-value decomposition (SVD) in combination with a band-pass filter to select respiratory frequencies [3] was used to compute a respiratory signal and used together with the ECG signal to sort the measured data into 6 respiratory and 16 cardiac motion bins. The highest two respiratory states were less densely sampled due to a higher variability in inhalation and discarded. In total this results in a scan efficiency of about 80% with the remaining 4 respiratory motion states.
2) Initial reconstruction using only data from the first 2:50 min (~45000 profiles). The respiratory state corresponding to the exhale position was reconstructed using Total-Variation in the cardiac phase dimension by minimizing $$\|PFSx - y\|^2_2 + \lambda TV(x),$$ see below for symbols. Averaging all cardiac phases, images for each respiratory motion state was reconstructed using five Conjugate-Gradient iterations.
3) Deformation vector fields (DVF) between three respiratory motion states and the exhale state were computed from the respiratory-resolved images by minimizing the mean-square-error between the morphed and the reference image using gradient-descent. DVF and inverse DVF were computed simultaneously and additionally regularized by a cycle loss and a quadratic gradient-penalty.
4) Motion-compensated reconstruction was performed based on all data available after 3:50 min (~60000 profiles). For this the cost function $$\sum_r \|PFST_rx - y_r\|^2_2 + \lambda TV(x)$$ is minimized using a version of the algorithm in Ref. [6] specialized to TV. Here, the final image $$$x$$$ contains the 3D volume and the cardiac phase dimension, $$$S$$$ represents the coil-sensitivity maps obtained from the Sense Reference scan (compressed to 8 coils [7]), $$$F$$$ the Fourier Transform, and the operator $$$T_r$$$ morphs $$$x$$$ into the image series corresponding to respiratory state $$$r$$$, and $$$y_r$$$ is the associated multi-coil, binned k-space data, which is measured at locations indicated by the projection operator $$$P$$$.
Results
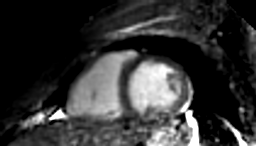
Cardiac cine images of the whole heart were reconstructed for all volunteers from in total 60000 profiles, i.e. the first 3:50 min of the acquired data. Total reconstruction time was less than 2 minutes, including reconstruction of initial images from 45000 profiles, computation of DVF, and the final non-rigid motion-compensated reconstruction. The contrast between myocardium and blood is sufficient for tissue delineation. The water-selective excitation successfully reduces signal from fatty tissue, which would otherwise degrade images acquired under free-breathing due to its high signal close proximity to the coils.Discussion
The potential for Whole-Heart Free-Breathing 3D Cardiac Cine MRI in a realistic setting has been demonstrated with an acquisition time of less than 4 minutes and a reconstruction delay of about one minute after completion of the acquisition. This could facilitate cardiac MRI with minimal planning and maximum patient comfort to be used routinely also outside of specialized imaging centers.Acknowledgements
No acknowledgement found.References
[1] Usman et al, "Free breathing whole-heart 3D CINE MRI with self-gated Cartesian trajectory", MRI, https://doi.org/10.1016/j.mri.2016.12.021
[2] Küstner et al, "Fully self-gated free-running 3D Cartesian cardiac CINE with isotropic whole-heart coverage in less than 2 min", NMR in Biomedicine, https://doi.org/10.1002/nbm.4409
[3] Sopra et al, "An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI", MRM, https://doi.org/10.1002/mrm.27898
[4] Doneva et al, ISMRM 2011, https://cds.ismrm.org/protected/13MProceedings/PDFfiles/0546.PDF
[5] Lin et al, "Rapid phase-modulated water excitation steady-state free precession for fat suppressed cine cardiovascular MR", JCMR, https://doi.org/10.1186/1532-429X-10-22
[6] Yao et al, "An efficient algorithm for dynamic MRI using low-rank and total variation regularizations", Medical Image Analysis, https://doi.org/10.1016/j.media.2017.11.003
[7] Kim et al, "Region-optimized virtual (ROVir) coils: Localization and/or suppression of spatial regions using sensor-domain beamforming", MRM, https://doi.org/10.1002/mrm.28706
Figures