1756
Self-calibrated through-time spiral GRAPPA for real-time, free-breathing evaluation of left ventricular function1Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2University of Michigan, Ann Arbor, MI, United States
Synopsis
Through-time non-Cartesian GRAPPA enables real-time, free-breathing evaluation of the left ventricle, but it requires extra calibration data. This work proposes a self-calibrated spiral GRAPPA method that uses an interleaved acquisition to eliminate the calibration scan. A long spiral trajectory is used to improve robustness to motion. Data were acquired at 12 LV slices with spatiotemporal resolutions of 2.08x2.08 mm2 and 32.72 ms/phase in 71 seconds. In a preliminary study of 6 volunteers comparing the proposed method to a gold-standard, functional values are within the limits of agreement in Bland-Altman analyses and are not statistically significantly different in paired t-tests (p<0.05).
Introduction
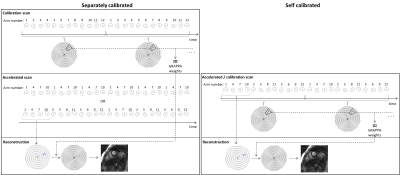
The goal of this work is to enable efficient real-time cardiac imaging to assess left ventricular function using a self-calibrated non-Cartesian GRAPPA approach. Through-time non-Cartesian GRAPPA has been demonstrated for free-breathing, real-time cardiac imaging (1,2). However, additional fully-sampled calibration data must be acquired at each slice, prolonging total scan time. It would be beneficial to instead derive GRAPPA weights from the undersampled data itself.In this work, we propose a self-calibrated through-time spiral GRAPPA method that uses an interleaved undersampling pattern, in which consecutive undersampled frames are merged to form fully-sampled calibration data. The standard non-Cartesian GRAPPA calibration and reconstruction processes can then be performed (Fig1). Hamilton et al previously presented a similar TGRAPPA-inspired method for radial trajectories (3,4), but errors were observed with rapid cardiac motion. These errors were likely due to motion over the merged undersampled frames and thus over the GRAPPA calibration kernels, yielding less accurate GRAPPA weights. In this work, a spiral trajectory was designed specifically for a self-calibrated acquisition, taking into consideration the time lapse over the GRAPPA kernels for improved reconstructions.
Methods
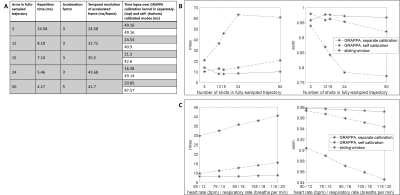
A spiral trajectory with a long readout was designed to reduce the number of arms needed to sample k-space, reduce the time lapse over GRAPPA calibration kernels, and improve robustness to motion. A range of trajectories were tested in digital phantom simulations (Fig2). The trajectory selected for in vivo testing has a TR of 8.18ms, and requires 12 arms to fully-sample k-space; data collected at R=3 (4/12 arms) provide a temporal resolution of 32.72ms/cardiac phase.Data were acquired in 6 healthy volunteers according to an IRB-approved protocol on a 1.5T scanner (Sola, Siemens Healthineers). The spiral multislice bSSFP sequence had the following parameters: TR/TE=8.18/0.93ms; field-of-view=300x300mm2; in-plane resolution=2.08x2.08mm2; slice thickness=8mm; 11-12 slices/stack; slice gap=25%; flip angle=58°-70°. Data for each slice were acquired sequentially, and all data were acquired under free-breathing and without ECG gating. A total of 180 undersampled frames were collected in 5.89 seconds/slice, yielding 60 fully-sampled frames for the self-calibrated reconstruction. The total time to acquire a stack of 12 slices was 71 seconds.
For comparison, a gold-standard breath-held, ECG-gated Cartesian cine scan was collected. The ranges of default imaging parameters over the volunteers were: field-of-view=255x340 to 375x378mm2; in-plane resolution=1.52x1.52 to 1.68x1.68mm2; slice thickness=8mm; 11-12 slices/stack; slice gap=20-25%; flip angle=57°-67°. Each slice was acquired in one breath-hold, and 25 cardiac phases were acquired at each slice. The temporal resolution was ~23-52ms/phase, depending on heart rate.
Spiral image reconstructions were performed in MATLAB. Cartesian cine data were evaluated using vendor-reconstructed DICOMs. All images were analyzed in the freely available software Segment (5). End systolic volume (ESV), end diastolic volume (EDV) and ejection fraction (EF) were calculated from manual segmentation of the endocardium. Bland-Altman plots and paired t-tests were prepared to compare the gold standard and proposed method.
Results
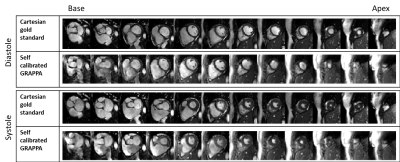
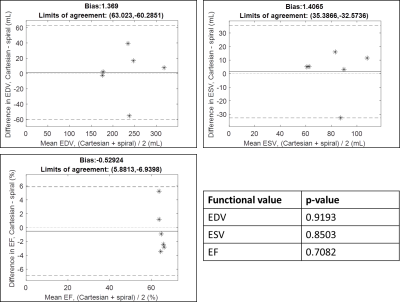
Fig2 shows a subset of the spiral trajectories tested in simulations. The 12-arm trajectory was predicted to be the most robust to motion and was tested in vivo.Fig3 shows a movie comparing the gold-standard cine scan and the proposed self-calibrated spiral method in a mid-ventricular slice in one volunteer. Fig4 shows still frames in diastole and systole from the full 12-slice stack in a second volunteer. Fig5 shows Bland-Altman comparisons between the gold standard and proposed methods for the functional values measured. Mean biases ([limits of agreement]) were 1.369mL ([63.02,-60.29]), 1.407mL ([35.39, 32.57]), and -0.5292% ([5.881,-6.940]) for EDV, ESV, and EF, respectively. 5/6 volunteers fell within the limits of agreement for the three values, while one volunteer was at the lower limit for ESV. In paired t-tests, the p-values for EDV, ESV, and EF were 0.9193, 0.8503, and 0.7082, respectively.
Discussion
These results suggest that a self-calibrated through-time spiral GRAPPA reconstruction can be used for efficient free-breathing, ungated imaging of the LV. By using a trajectory designed specifically for this reconstruction, the sensitivity to motion of the self-calibration scheme was reduced.Bland-Altman plots show that functional values calculated using the proposed method are within the limits of agreement with the gold-standard. While the limits are wider than reported in prior works using through-time non-Cartesian GRAPPA for LV evaluation (1,2), in this small number of healthy volunteers the EF values for both the Cartesian and spiral scans are within a normal range. (6) suggests that when comparing different imaging modalities to calculate EF, an absolute difference in EF of <5% may be considered agreement. In this work, the largest absolute difference was 5.23%, and the mean absolute difference was 2.67%.
Next steps include segmentation and image quality ratings by trained cardiologists to better compare the proposed method to the gold standard.
Conclusion
This work presents an efficient approach for real-time, free-breathing cardiac imaging using self-calibrated through-time spiral GRAPPA. Undersampled data are acquired with a specially designed spiral trajectory using an interleaved arm order, and consecutive frames are merged to form fully-sampled calibration data, eliminating the need for a separate calibration scan. This method enables full left ventricular coverage in 71 seconds of free-breathing, ungated scanning. In a preliminary study of 6 healthy volunteers, functional values are not statistically significantly different from gold-standard, ECG-gated breath-held scans (p < 0.05).Acknowledgements
No acknowledgement found.References
1. Aandal G, Nadig V, Yeh V, et al. Evaluation of left ventricular ejection fraction using through-time radial GRAPPA. J. Cardiovasc. Magn. Reson. 2014;16:1–13.
2. Barkauskas KJ, Rajiah P, Ashwath R, et al. Quantification of left ventricular functional parameter values using 3D spiral bSSFP and through-time non-Cartesian GRAPPA. J. Cardiovasc. Magn. Reson. 2014;16:1–13.
3. Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA). Magn. Reson. Med. 2005;53:981–985 doi: 10.1002/mrm.20430.
4. Hamilton JI, Wright KL, Griswold MA, Seiberlich N. Self-Calibrating Interleaved Reconstruction for Through-Time Non-Cartesian GRAPPA. In: Proceedings of the 13th Annual Meeting of the International Society for Magnetic Resonance in Medicine. Salt Lake City; 2013. p. 3836.
5. Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med. Imaging 2010;10 doi: 10.1186/1471-2342-10-1.
6. Pellikka PA, She L, Holly TA, et al. Variability in Ejection Fraction Measured by Echocardiography, Gated Single-Photon Emission Computed Tomography, and Cardiac Magnetic Resonance in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. JAMA Netw. Open 2018;1 doi: 10.1001/jamanetworkopen.2018.1456.
7. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020;22:1–18 doi: 10.1186/s12968-020-00607-1.
8. Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease : SCMR expert consensus group on congenital heart disease. J. Cardiovasc. Magn. Reson. 2013;15:1–26.
Figures