1754
Deep artifact suppression for interventional cardiac MR-thermometry1Institute of Cardiovascular Science, University College London, London, United Kingdom, 2Electrophysiology and Heart Modeling Institute, Foundation Bordeaux Université, IHU Lyric, Bordeaux, France, 3Centre de recherche Cardio-Thoracique de Bordeaux, Universite Bordeaux, Bordeaux, France, 4Centre de recherche Cardio-Thoracique de Bordeaux, INSERM, Bordeaux, France, 5Centre de Résonance Magnétique des Systèmes Biologiques, Bordeaux, France
Synopsis
A novel thermometry acquisition and a fast deep learning based image reconstruction were combined for cardiac interventional thermometry at high spatial (0.86×0.86mm2) and temporal (0.97s) resolutions, robust to motion and susceptibility artefact and independent of external ECG-gating. The method was tested in phantom and in-vivo in a sheep. The proposed deep learning method outperformed the state-of-the-art algorithm in terms of SNR and paves the way for clinical studies.
Introduction
Thermal ablation uses heat or cold energy to damage tissue. It is used in the heart to block abnormal electrical signals, which cause arrythmias, and restore a normal heartbeat. Cardiac MR temperature imaging allows non-invasive, real-time monitoring of the cardiac muscle temperature and lesion formation during the procedure1. Continuous radial golden angle Gradient-Echo (GRE) acquisition combined with intra-scan motion correction2 could be a robust and user-friendly alternative allowing to overcome the limitations (low temporal resolution and susceptibility artefacts) of Gradient-Echo (GRE)3 or Echo Planar Imaging (EPI)4.However, it requires to highly accelerate data acquisition to achieve the required sub-second temporal resolution. To recover good quality images from such accelerations (10 to 20x) advanced reconstruction methods such as Compressed Sensing are required but usually have prohibitively long reconstruction times for real time monitoring.
Here we propose a fast machine learning (ML) deep artifact suppression method, to enable rapid reconstruction of complex images from 10x undersampled data5. The high-quality reconstructed images are then used to perform MR thermometry, and the resulting temperatures are compared to the current state of the art method with direct temperature estimation from undersampled k-space data6.
Methods
Continuous GRE radial golden angle acquisitions were used to monitor RF ablations in an agar-gel phantom and in-vivo in a sheep heart (protocol approved by ethics committee). An MR-compatible radiofrequency ablation (RFA) catheter (Vision-MR Ablation Catheter, Imricor Medical Systems, Burnsville, MN, USA) with two embedded micro-coils (proximal and distal) was used to perform the ablation.In-vivo, a 2D intra-scan rigid motion correction based on concomitant catheter signal tracking was performed, coupled with non-rigid registration of magnitude and phase for the residual motion. The RFA catheter was inserted in the femoral artery and navigated through the left ventricle under MRI guidance using active tracking via the prototype Monte Carlo platform (Siemens Healthcare, Erlangen, Germany).
Acquisition parameters for real-time imaging during thermal ablation were: FOV=220×220mm2, resolution=0.86×0.86mm2, slice thickness 3 mm, TR=24.14ms, TE=20ms, FA=15o. Temperature maps were reconstructed using two different methods (Fig.1): (1) direct estimation (Direct Estim) from undersampled k-space data combined with a hybrid multi-baseline referenceless treatment5 of the image model correcting for susceptibility induced phase variation due to organ motion. (2) NUFFT followed by fast deep artifact suppression (see below) associated with model-based phase subtraction3. In both cases the temporal resolution was set to 0.97s by using 40 consecutive spokes. To assess the precision of MR thermometry, the temporal standard deviation of temperature σ(T) was computed in each pixel before heating.
Deep artifact suppression was performed using a U-Net similar to 5, trained using 520 breath-hold gated spiral aortic flow complex datasets. Synthetic paired training data was created by emulating the thermometry data acquisition, i.e. adding of background phase, noise (target SNR=10) and simulating the sampling. Training was performed using an average SSIM loss5, Adam optimizer, 50 epochs and learning rate of 5E-4. Deep artifact suppression is performed in a sliding window fashion with a step size of 20 and window size of 40 frames where only the central 20 frames are kept.
Results
The ML deep artifact suppression successfully reconstructed thermometry images both in-vivo and on a phantom with an acceleration factor of 10 (Fig. 2,3,4). The U-Net, trained from aortic flow datasets, showed excellent generalizability to unseen geometries and data.Ex-vivo experiment: an increase of σ(T) (spatial mean ± standard deviation over the ROIs) values were observed for the deep artifact suppression reconstruction with a mean value of 0.9 ±0.5°C versus 0.6±0.1°C for the direct estimation method. Both values are acceptable in view of typical temperature reached in tissue near the catheter tip (>15°C temperature increase). The resulting temperature evolution curves exhibit a higher SNR compared to the one obtained with direct estimation (Fig. 2).
In-vivo experiment: NUFFT followed by deep artifact suppression combined with model-based phase subtraction showed consistent evolution of the tissue temperature near the catheter (Fig. 4), with a σ(T) before heating close to 1°C at the vicinity of the catheter tip. A maximal temperature increase of 17 °C at the catheter tip can be observed. Fig 5 shows an animated movie during the ablation.
The proposed method was trained from aortic flow DICOM data collected during clinical routine. It therefore did not require assembling a thermometry specific dataset and could reduce likeliness of observing “fake heating” artefacts compared to a model trained using thermometry data.
Additionally, the proposed reconstruction is extremely fast (on average 42 ms per frame: 21.5ms for NUFFT and 20.5ms for deep artifact suppression) paving the way for real-time acquisition and visualisation.
Conclusion
We propose a method allowing interventional cardiac thermometry at high spatial (0.86×0.86mm2) and temporal (0.97s) resolutions, robust to motion and susceptibility artefact and independent of external ECG-gating. The proposed ML method outperformed the state-of-the-art algorithm in terms of SNR and paves the way for clinical studies when implemented in real-time.Acknowledgements
This work was supported by the British Heart Foundation (grant: NH/18/1/33511), the National Research Agency IHU-LIRYC (ANR-10-IAHU04-LIRYC) and CARTLOVE (ANR-17-CE19-0007).
We thank Dr. Solenn Toupin (Siemens Healthcare) and Dr. Wadie Ben Hassen (Siemens Healthcare) for their help during the in vivo acquisitions. We thank Tom Lloyd (Imricor Medical Systems) and Jason Stroup (Imricor Medical Systems) for help and guidance using the Advantage MR system.
References
1 Toupin, S. et al. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J. Cardiovasc. Magn. Reson. 19, 1–12 (2017).
2 Yon M. et al. Continuous cardiac thermometry via simultaneous catheter tracking and undersampled radial golden angle acquisition. Submitted to Proceedings of the ISMRM, London (2022).
3 Kolandaivelu, A. et al. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using MRI thermography. Circ. Arrhythmia Electrophysiol. 3, 521–529 (2010).
4 Ozenne, V. et al. Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation. Magn. Reson. Med. 77, 673–683 (2017).
5 Jaubert O, et al. Deep artifact suppression for spiral real-time phase contrast cardiac magnetic resonance imaging in congenital heart disease. Magn. Reson. Imaging. 2021 Nov; 83:125-132. (2021)
6 Gaur, P. & Grissom, W. A. Accelerated MRI thermometry by direct estimation of temperature from undersampled k-space data. Magn. Reson. Med. 73, 1914–1925 (2015).
Figures
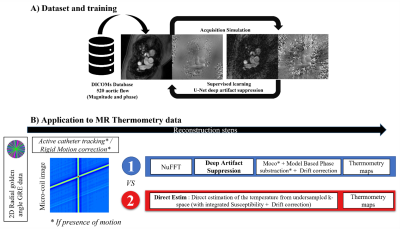
Figure 1: Schematic block diagram of the reconstruction workflow. A) A U-Net was trained for deep artifact suppression using 520 breath-hold gated spiral aortic flow datasets. A simulated radial golden angle sampling was applied to emulate the thermometry data. B) Thermometry images were reconstructed using two different methods: (1) NUFFT followed by deep artifact suppression. (2) Direct estimation (Direct Estim) of the temperature from undersampled k-space. Additional motion correction processing was integrated before and after reconstruction for the in-vivo dataset.

Figure 2: Performance of the reconstructions on a static agar gel dataset. A) GRE-radial golden angle image of a gel phantom with an MR compatible catheter. B) Magnitude image, C) temporal standard deviation temperature maps (or temperature uncertainty) before heating, D) temperature maps during heating Direct Estimation (left) versus U-Net (right). E) Distribution of the standard deviation of the temperature evolution in time. F) Temporal evolution of the temperature in a 3x3 kernel of voxels (White Square). RF Power (10 W) is turned on and off, at 2 and 3min respectively.
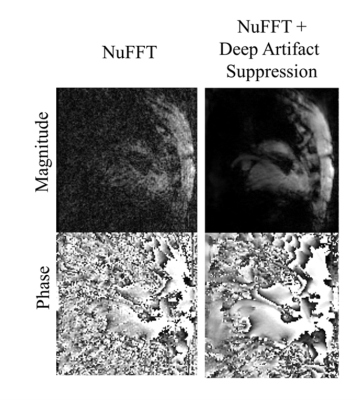
Figure 3: Image quality in-vivo: GRE-radial golden angle magnitude and phase images of a sheep heart during intervention obtained after NUFFT (input to the network) and following deep artifact suppression.
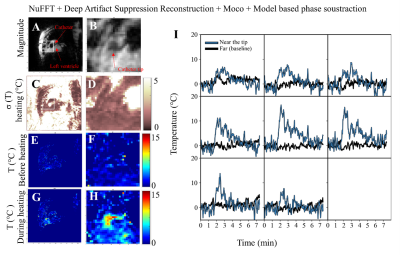
Figure 4. In-vivo case results using the deep artifact suppression method. (A,B) Full view and zoom view of magnitude image, (C,D) temporal standard deviation temperature maps (or temperature uncertainty) before heating, (E,F) temperature maps before heating, (G,H) temperature maps during heating. RF POWER (30W) was applied between 1min30 and 2min30. (I) Temporal evolution of the temperature in a 3x3 kernel of voxels near the tip of the catheter. Black line depicts baseline temperature outside heating zone.
Figure 5. Animated movie of the in-vivo ablation (shown at 10x the acquisition speed). (Left) Motion corrected magnitude images. (Middle) Zoom view of the same magnitude images, (Right) Zoom view of the motion corrected temperature maps (in oC). The animation is available with better definition at this link: https://www.rmsb.u-bordeaux.fr/rmsbcloud/s/mmJADd26JN3Tzm5