1748
THE ASSOCIATION BETWEEN PLAQUE SURFACE DISRUPTION AND FIBROUS CAP STATUS AT BASELINE AND VOLUME CHANGE OF INTRAPLAQUE HEMORRHAGE OVER TWO YEARS1CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands, Maastricht, Netherlands, 2Department of Radiology and Nuclear Medicine, Maastricht University medical center (MUMC+), maastricht, Netherlands, 3Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands, 4Department of Neurology, Amsterdam UMC, location AMC, Amsterdam, Amsterdam, Netherlands, 5Division of Image Processing, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 6Radiology, University Medical Center Utrecht, Utrecht, Utrecht, Netherlands, 7Department of Clinical Neurophysiology, Maastricht University Medical Center+ (MUMC+), maastricht, Netherlands, 8Epidemiology, Erasmus MC, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands, 9Department of Epidemiology, Maastricht University, Maastricht, The Netherlands, maastricht, Netherlands, 10Department of Neurology, Maastricht University Medical Centre, maastricht, Netherlands
Synopsis
The factors that contribute to intraplaque hemorrhage (IPH) development within the carotid atherosclerotic plaque are incompletely understood. Previously, we demonstrated that IPH is associated with a thin/ruptured fibrous cap (TRFC) and disruption of the plaque surface on in a cross-sectional study. Baseline and 2 year’s follow-up carotid MR images of 110 patients from the Plaque at Risk (PARISK) study were analyzed. IPH volume change in TRFC at baseline was significant different (p=0.04) than thick fibrous cap group. Baseline IPH volumes are larger in patients with TRFC and disrupted plaque surface, but didn’t increase during the follow-up.
Background
Intraplaque hemorrhage (IPH) is an important characteristic of the vulnerable plaque and a strong predictor for stroke (1). The factors that contribute to IPH development within the carotid atherosclerotic plaque are incompletely understood. Previously, we demonstrated that IPH is associated with a thin/ruptured fibrous cap (TRFC) on Magnetic Resonance Imaging (MRI) and disruption of the plaque surface on Computed Tomography Angiography (CTA) in a cross-sectional study.Aim
In the present study, we aim to investigate the relationship between thin/ruptured fibrous cap and disruption of the plaque surface and the volume change of IPH over a two-year period.Methods
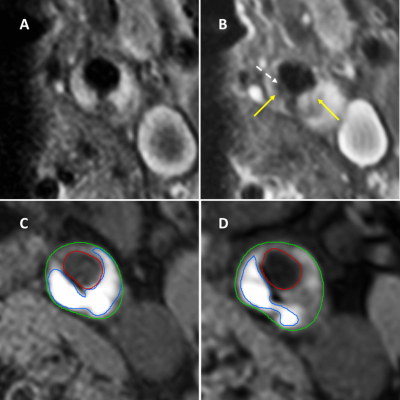
Stroke patients with ≥2-3 mm ipsilateral carotid plaque and <70% stenosis were included in the Plaque at Risk (PARISK) study (2). 127 and 97 of these patients underwent baseline carotid MRI and CTA, respectively, and follow-up MRI after two years. IPH and the fibrous cap status on MRI and disruption of the plaque surface (e.g. ulceration or a fissured fibrous cap) on CTA were delineated/scored by trained observers based on previously described criteria (2-5). a Chi-Square test was used to investigate the association between thin or ruptured fibrous cap/ disruption of plaque surface and the presence of IPH at baseline. Multivariate logistic regression models with the presence of IPH at the end of follow-up as dependent variable was used to study the correlation between thin or ruptured fibrous cap/ disruption of plaque surface and IPH volume change over two years.Results
In total for 110 and 93 ipsilateral carotid arteries the FC status on MRI and plaque surface on CTA could be scored, respectively. A strong positive association was found for TRFC (OR: 19.2, 95% CI: 7.2-51.1, p<0.05) and disruption of the plaque surface (OR:3.7, 95% CI: 1.5-8.8, p= 0.004) with the presence of IPH at baseline. There were significant differences when comparing the IPH volume changes over two years between the subgroups (TRFC at baseline: median -2.1 mm3, thick FC at baseline: 0 mm3, p=0.04) (Figure 1). the IPH volume didn’t change significantly (p=0.2) after 2 years of follow-up between patients with a smooth plaque surface or disrupted plaque surface. A significant correlation was found between the baseline fibrous cap status and IPH volume change over two years (OR: 5.7; 95% CI: 2.1 – 15.3, p< 0.05).Conclusion
Patients with TRFC and plaque surface disruptions have larger IPH volumes at baseline compared to patients with thick FC or smooth plaque surface. Patients with TRFC at baseline showed regression in IPH volume over two years compared to patients with thick FC. A similar finding was observed when comparing IPH volume changes in patients with and without disruption of the plaque surface, however, this finding was not statistically significant. In conclusion, although baseline IPH volumes are larger in patients with a thin or ruptured fibrous cap and disrupted plaque surface, in the majority of the patients the volume of IPH does not further increase during 2 years of follow-up.Acknowledgements
No acknowledgement found.References
1.Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):395-406.
2.Truijman MT, Kooi ME, van Dijk AC, de Rotte AA, van der Kolk AG, Liem MI, et al. Plaque At RISK (PARISK): prospective multicenter study to improve diagnosis of high-risk carotid plaques. International journal of stroke: official journal of the International Stroke Society. 2014;9(6):747-54.
3. Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107(24):3047-52.
4. Kwee RM, van Engelshoven JM, Mess WH, ter Berg JW, Schreuder FH, Franke CL, et al. Reproducibility of fibrous cap status assessment of carotid artery plaques by contrast-enhanced MRI. Stroke. 2009;40(9):3017-21.
5. Lovett JK, Gallagher PJ, Hands LJ, Walton J, Rothwell PM. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation. 2004;110(15):2190-7.
Figures