1742
Simultaneous PET/fMRI of the Kappa Opioid Receptor System During Psychotropic Drug Challenge1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Synopsis
This research utilizes simultaneous PET/MR imaging to provide a preliminary analysis of the kappa-opioid receptor (KOR) system in a non-human primate (NHP) brain. We investigated the influence of a psychoactive drug challenge specific to the KOR system in a NHP using the KOR agonist Salvinorin-A. We report regionally specific uptake of a KOR agonist radiotracer ([11C]EKAP) and alterations to BOLD-derived cerebral blood volume (CBV) following the use of Salvinorin-A that are directly related to radiotracer uptake distribution. This work serves a preliminary foundation for KOR imaging and understanding KOR-agonist drug mechanisms through PET/MR.
Introduction
Like other members of the opioid receptor family, the kappa opioid receptor (KOR) promotes antinociception and influences psychoactive behavior. However, drugs that are KOR agonists are unique in that they don’t induce euphoria by elevating dopamine levels like other abused substances1. Moreover, psychoactive KOR agonists may provide a therapeutic opportunity for diseases influencing mental perception, such as schizophrenia, dementia, etc.2, but their physiological effects are not fully understood. In PET imaging, KOR agonist radiotracers, like [11C]EKAP, have optimal pharmacokinetics and adequate binding to this receptor system3 and has been used in humans previously4. Simultaneous PET/fMRI of the KOR system provides an opportunity to relate neurochemical phenomena to functional hemodynamic changes relevant to KOR pathways5. This preliminary study utilizes simultaneous PET/fMRI to evaluate the influence of a potent psychotropic KOR agonist, Salvinorin-A, on functional cerebral blood volume (CBV) and KOR occupancy in a non-human primate (NHP).Methods
Imaging was performed on a 3T Siemens TIM-Trio with a BrainPET insert with a custom 8-channel head coil. An NHP was anesthetized and maintained at ~1.0-1.2% isofluorane. A 1mm isotropic T1-weighted MPRAGE (TE1/TE2/TE3/TE4 = 1.64/3.5/5.36/7.22 ms, TR = 2530 ms, TI = 1200 ms, flip angle = 7°) was acquired as an anatomical image. For fMRI, the NHP was injected with Ferumoxytol (MION; 10mg/kg) during a EPI sequence (TE/TR = 22/3000ms, 1.3mm isotropic resolution), 100 repetitions. An additional EPI sequence was then performed with simultaneous PET imaging for 100 minutes with 2000 repetitions. [11C]EKAP3 was intravenously given as a bolus injection (3.5mCi) with continuous infusion (1.5mCi) to achieve steady-state radiotracer uptake. After 40-min., an I.V. injection of Salvinorin-A (4μg/kg) was performed. fMRI images were motion corrected, brain extracted, and affine registered to an NHP brain template along with PET data and assessed for neurochemical and physiological changes. PET data was binned into 100 frames and analyzed for [11C]EKAP radioactivity (Bq/mL) to quantify receptor binding/occupancy over the time-course. fMRI signal was used to calculate %CBV as:%CBV = ln(Sstim/Sctrl)/ln(Spre-MION/Sctrl) [Eqn. 1]
Sstim is fMRI signal during drug challenge, Spre-MION is pre-MION signal6, and Sctrl is post-MION signal prior to drug challenge. ln(Sstim/Sctrl) was estimated by fitting fMRI data to a gamma function for fast and slow response to Salvinorin-A. Percent receptor occupancy was calculated from PET data as:
%O = (Bq/mLpre-drug - Bq/mLpost-drug)/Bq/mLpre-drug [Eqn. 2]
Results and Discussion
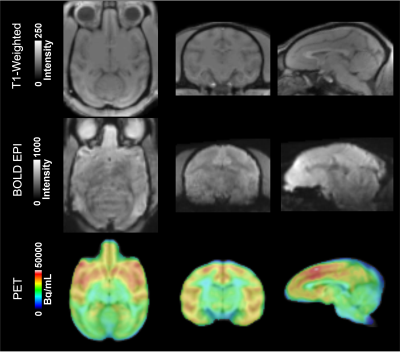
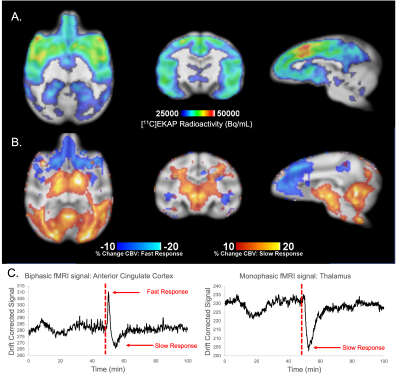
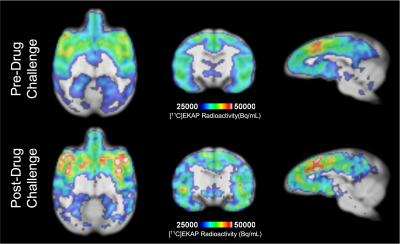
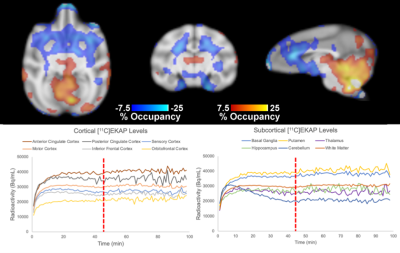
T1-weighted structural, EPI, and reconstructed PET data were successfully registered to an NHP brain template (Figure 1). Regional [11C]EKAP radioactivity (Bq/mL) distributions prior to Salvinorin-A administration are observed in PET data (Figure 2A). Higher [11C]EKAP uptake correlates to higher KOR densities with known distribution as described previously3. fMRI signal profiles during drug challenge were found to be best described by a monophasic or biphasic gamma function delineating fast/slow hemodynamic responses (Figure 2C). Changes in %CBV exceeding +/-10% following Salvinorin-A injection are represented in Figure 2B, clearly indicating regionally specific alterations in CBV that correspond to [11C]EKAP distribution and radioactivity levels.Average radiotracer uptake may increase in KOR rich regions following Salvinorin-A administration (Figure 3A vs. Figure 3B). Post-hoc assessment of %O (Figure 4) appears to match the trends of %CBV. Sample radiotracer uptake profiles are included for reference (Figure 4B). These results may indicate that Salvinorin-A elevates KOR uptake levels following receptor activation in high density regions, potentially by modulating receptor affinity or depleting endogenous opioid peptides. In fact, the relationship between receptor occupancy and peak %CBV changes have been described in previous PET/fMRI work7. The presented work further highlights potential neurovascular coupling to receptor occupancy as different receptor systems may show different occupany:function relationship. Additional pharmacokinetic analysis with a larger animal cohort will be conducted to confirm this.
Summarily, functional changes from Salvinorin-A are reported throughout the NHP brain. The degree of fast hemodynamic response depends on initial KOR density and uptake, whereas slow responses occur throughout the brain but with peak changes in less KOR rich regions, potentially representing secondary responses from other receptors connected to the KOR pathway. For more quantitative measures of receptor affinity and drug occupancy, follow up work of the [11C]EKAP and Salvinorin-A relationship will be pursued by kinetic modeling of PET data to a suitable reference region void of KORs. Results will be compared to the CBV-fMRI responses highlighted here.
Conclusion
This preliminary work demonstrates the utility of simultaneous PET/fMRI for investigating correlations of hemodynamic response to KOR occupancy. Salvinorin-A may dramatically influence regional functional activity and potentially alter radiotracer uptake. As such, Salvinorin-A induced CBV changes may be best represented as a biphasic gamma function with delineation between fast and slow responses that correlate well with radiotracer distribution. Additional experiments in a larger cohort of NHPs will verify this phenomenon and its statistical significance, and the scope of analysis will be expanded to include receptor binding potential to identify if Salvinorin-A alters receptor affinity after activation.Acknowledgements
This work is supported by pilot Funding from the Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital.References
1. Al-Hasani, R., & Bruchas, M. R. (2011). Molecular Mechanisms of Opioid Receptor-Dependent Signaling and Behavior. Anesthesiology, 115(6), 1363.2.
2. Roth, B. L., Baner, K., Westkaemper, R., Siebert, D., Rice, K. C., Steinberg, S. A., Ernsberger, P., & Rothman, R. B. (2002). Salvinorin A: A potent naturally occurring nonnitrogenous κ opioid selective agonist. Proceedings of the National Academy of Sciences of the United States of America, 99(18), 119343.
3. Naganawa, M., Li, S., Nabulsi, N., Lin, S. F., Labaree, D., Ropchan, J., Gao, H., Mei, M., Henry, S., Matuskey, D., Carson, R. E., & Huang, Y. (2020). Kinetic Modeling and Test–Retest Reproducibility of 11C-EKAP and 11C-FEKAP, Novel Agonist Radiotracers for PET Imaging of the κ-Opioid Receptor in Humans. Journal of Nuclear Medicine, 61(11), 1636–1642.4.
4. Martinez, D., Slifstein, M., Matuskey, D., Nabulsi, N., Zheng, M. Q., Lin, S. fei, Ropchan, J., Urban, N., Grassetti, A., Chang, D., Salling, M., Foltin, R., Carson, R. E., & Huang, Y. (2019). Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study. Neuropsychopharmacology, 44(10), 1720.5.
5. Wey, H. Y., Catana, C., Hooker, J. M., Dougherty, D. D., Knudsen, G. M., Wang, D. J. J., Chonde, D. B., Rosen, B. R., Gollub, R. L., & Kong, J. (2014). Simultaneous fMRI-PET of the Opioidergic Pain System in Human Brain. NeuroImage, 102(0 2), 275.6.
6. Shih, Y. Y. I., Wey, H. Y., de La Garza, B. H., & Duong, T. Q. (2011). Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. Journal of Cerebral Blood Flow & Metabolism, 31(3), 832.7.
7. Sander, C. Y., Hooker, J. M., Catana, C., Normandin, M. D., Alpert, N. M., Knudsen, G. M., Vanduffel, W., Rosen, B. R., & Mandeville, J. B. (2013). Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 110(27), 11169–11174.
Figures