1740
Evaluating cerebrovascular reactivity dynamics through a Bayesian inference approach
Joana Pinto1, Martin Craig2,3, Paula Croal2,3, Nicholas P. Blockley2,4, Michael A. Chappell2,3, and Daniel P. Bulte1
1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Sir Peter Mansfield Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 3Radiological Sciences, Mental Health & Clinical Neurosciences, School of Medicine, Nottingham, United Kingdom, 4School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Sir Peter Mansfield Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 3Radiological Sciences, Mental Health & Clinical Neurosciences, School of Medicine, Nottingham, United Kingdom, 4School of Life Sciences, University of Nottingham, Nottingham, United Kingdom
Synopsis
Cerebrovascular reactivity (CVR) has been shown to be an important parameter that is altered in certain pathologies. Analysis strategies using MRI data typically assume similar responses across the brain, neglecting the need and potential of modelling temporal features. In this work, we propose and test a novel method for the evaluation of CVR dynamics based on a variational Bayesian approach. Although this method yields similar CVR results to more standard approaches, it is more time efficient and has the potential to improve modelling by incorporating non-linear and biophysically informed models.
Introduction
Cerebrovascular reactivity (CVR) can be defined as the mechanism of cerebral blood vessels to adjust their calibre and cerebral blood flow (CBF) in response to a vasoactive stimulus (e.g., hypercapnia)1. Although there are several ways to evaluate CVR using MRI2,3, most modelling strategies use a single model to explain CBF changes across the brain, only evaluating amplitude changes (CVRamp) and thus overlooking any variations in temporal features (CVRdelay). One way to include and/or infer timing variations in the modelling is by applying iterative general linear model (GLM) fitting using time-shifted regressors, but this is highly dependent on the range of shifts tested. Another solution is to perform a Fourier basis analysis, but this strategy can only be performed if MR data and stimuli follow a sinusoidal waveform. In this work, we propose and test an alternative method to evaluate CVR dynamics based on a non-linear model fit using variational Bayesian inference (VB)4. We compare this novel approach with more standard methods such as time-shifted GLM (GLMshift)5 and Fourier-basis modelling (GLMsine)6. The VB strategy has the added benefits of estimating CVR amplitude and temporal information in a single model fit, and, by allowing incorporation of prior information based on physiological knowledge, increasing model fitting stability, and enhancing the interpretability of results.Methods
A variational Bayesian inference approach4 for CVR mapping was applied within the software Quantiphyse (www.quantiphyse.org). Bayesian statistics provide a generic framework for the adjustment of belief in an event in the presence of new information (prior information) and the variational method facilitates analytical calculations arising in Bayesian inference.BOLD-fMRI CVR data from ten healthy subjects (5M, 20.4 ± 0.8 years old) were acquired on a 3T Siemens scanner, using a GE-EPI sequence (TR/TE = 2/0.03s, 24 slices, 3.5x3.5x =5 mm3), during a sinusoidally modulated hypercapnia challenge (seven sinusoidal cycles, period of 60s each; variation in end-tidal CO2 (PetCO2) from resting baseline to +10mmHg).
MR data underwent standard pre-processing using FSL’s FEAT (brain extraction, slice timing correction, distortion correction, motion correction, temporal filtering, spatial smoothing) and subsequent analysis was done in Quantiphyse and MATLAB. Data modelling was performed using three strategies: variational Bayesian inference (VB), time shifted GLM (GLMshift), sinusoidal GLM (GLMsine). For VB, the corresponding processed PetCO2 trace was used as model, applying a total of 80 iterations and setting parameter priors as follows: gaussian probability distributions; CVRamp: mean = 1, std = 2000 and CVRdelay = mean 0, std = 100. For GLMshift, the corresponding processed PetCO2 trace was also used as a model and a total of 60 shifts were tested (-30s to 30s in steps of 0.2s; for consistency/comparison purposes). Additional ranges and steps were also tested for this approach. For GLMsine, a single GLM was performed using a sine and a cosine wave (period 60s) as regressors. In all strategies, the estimated parameters of interest were CVR amplitude (CVRamp in %/mmHg) and timing (CVRdelay in seconds).
Results and Discussion
Fig 1 displays illustrative CVR maps of all model fittings tested (VB, GLMshift, GLMsine) for 3 different subjects. The spatial patterns of the CVRamp maps obtained are similar across the methods tested, except for some voxels around the edges of the brain and ventricles (low SNR). Nevertheless, the GLMsine yielded lower median CVRamp values across GM (Fig 2), possibly due to an overall poorer fit of this strategy given that the measured BOLD response was not completely sinusoidal. The CVRdelay maps were more variable across methods, particularly in the regions with low SNR (Fig 3). Additionally, the GLMshift was highly dependent on the range of shifts tested (Fig 4) with some voxels yielding large and, sometimes unrealistic, delays, as highlighted in the model fitting of specific voxels in Fig 3. In contrast, the CVRdelay maps obtained with the VB and GLMsine approaches were more homogeneous, although VB was also dependent on the initial alignment between PetCO2 and BOLD.Conclusion
In this work we introduce a Bayesian method that models BOLD MR data and estimates CVRamp and CVRdelay simultaneously in a single step. This method provides similar results to more standard approaches and has the potential to improve CVR modelling by allowing incorporation of prior information based on physiological knowledge and the possibility of inclusion of non-linear and possibly more realistic models, such as the sigmoidal relationship between BOLD and PetCO27 (future work).Acknowledgements
This work was funded by EPSRC grants EP/G004277/1, EP/K025716/1, EP/S021507/1 and EP/P012361References
- Liu P, et al. (2019) Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review, Neuroimage; 187: 104–115.
- Pinto J, et al. (2021) Cerebrovascular Reactivity Mapping Without Gas Challenges: A Methodological Guide, Front. Physiol; 11:608475.
- Sleight E, et al. (2021) Cerebrovascular Reactivity Measurement Using Magnetic Resonance Imaging: A Systematic Review, Front. Physiol; 12:643468.
- Chappell MA, et al. (2009) Variational Bayesian Inference for a Nonlinear Forward Model," IEEE Transactions on Signal Processing; 57, 1: 223-236.
- Moia S, et al. (2020) Voxelwise optimization of hemodynamic lags to improve regional CVR estimates in breath-hold fMRI, 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, 1489-1492
- Pinto J, et al. (2016). Fourier modeling of the BOLD response to a breath-hold task: Optimization and reproducibility, Neuroimage; 15,135: 223-31.
- Bhogal AA, et al. (2014) Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage; 98: 296-305
Figures
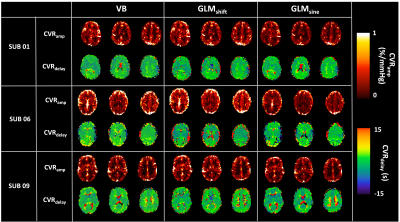
Figure 1. Illustrative example of CVRamp (top row) and CVRdelay (bottom row) maps obtained with the three strategies tested, variational Bayesian (VB), time shifted GLM (GLMshift), sinusoidal GLM (GLMsine), for three representative subjects.
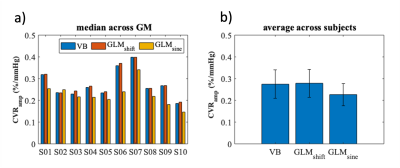
Figure 2. (a) Median CVRamp values of each subject for the three strategies tested (variational Bayesian (VB), time shifted GLM (GLMshift), sinusoidal GLM (GLMsine)) across GM. (b) Average CVRamp values across subjects for each one of the modelling approaches. Error bars represent standard deviation.
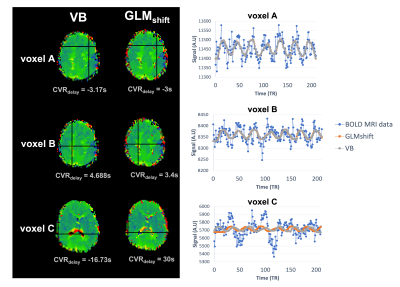
Figure 3. (left) Example of CVRdelay maps of subject S01 obtained for the variational Bayesian (VB) and time shifted GLM (GLMshift) approaches and (right) the corresponding model fittings of specific voxels (A, B and C), selected to highlight differences and similarities between approaches.
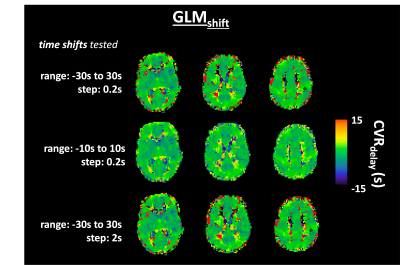
Figure 4. Illustrative example of CVRdelay maps for subject S09 obtained with the GLMshift approach and using different time-shift ranges and steps.
DOI: https://doi.org/10.58530/2022/1740