1715
Bipolar Partial Echo Imaging
Holger Eggers1 and Jochen Keupp1
1Philips Research, Hamburg, Germany
1Philips Research, Hamburg, Germany
Synopsis
Partial echo imaging is widely used in angiography to shorten the echo time and reduce flow-induced signal loss. However, sampling only an asymmetric part of k-space basically ignores high-frequency phase and thus causes signal alteration as well. In the present work, bipolar partial echo imaging is introduced to cover the full k-space sparsely with a Cartesian partial echo measurement. It involves reversing the polarity of the readout gradient during the acquisition and recovering missing data in the reconstruction without assuming smoothness of the phase. The potential of bipolar partial echo imaging is demonstrated in phantom and first volunteer experiments.
Introduction
Partial Fourier imaging commonly covers only an asymmetric part of k-space in the readout or phase encoding direction. It permits a reconstruction of real signal and even of complex signal with low-frequency phase, but information on high-frequency phase is essentially lost.1 Since the part of k-space omitted in the acquisition is large and contiguous, a reconstruction based on compressed sensing (CS) or parallel imaging (PI) generally also fails in accurately estimating the missing data.2,3 In the present work, conventional unipolar partial echo imaging is turned into bipolar partial echo imaging. Instead of densely sampling only a part of k-space, bipolar partial echo imaging allows sparsely sampling the full k-space with a Cartesian partial echo measurement, as shown in Fig. 1, and thus capturing high-frequency phase. In this way, it promises to resolve artifacts associated with unipolar partial echo imaging, such as signal loss.Methods
The modified spoiled gradient-echo sequence illustrated in Fig. 2 is used to explore bipolar partial echo imaging. In half of the repetitions, the polarity of the gradients in readout direction is reversed. Since the switching interrupts the spoiling, it is preferably performed only once. Alternatively, the spoiling is maintained by keeping the polarity of the trailing spoiler gradient and increasing the area such that the integral of the gradients in readout direction remains constant in each repetition. This permits interleaving the two subacquisitions with positive and negative polarity of the readout gradient.Instead of assuming smoothness of the phase, as in homodyne reconstruction, a combined CS-PI reconstruction is employed to synthesize the missing data. However, inconsistencies between the two subacquisitions arise from spatial variations of the main magnetic field B0 and from eddy current-induced phase errors. They are addressed by integrating distortions and phase errors into the forward model of the reconstruction $$y=M_1 F D_1 C P_1 x + M_2 F D_2 C P_2 x ,$$ where $$$x$$$ and $$$y$$$ are the unknown image and the measured data, $$$P_n$$$ are diagonal phase matrices, $$$C$$$ is the coil sensitivity matrix, $$$D_n$$$ are sparse distortion matrices, $$$F$$$ is the Fourier transform matrix, $$$M_n$$$ are masks, and $$$n$$$ is the index distinguishing the two subacquisitions.
Experiments were performed on a 3 T Ingenia Elition scanner (Philips, Best, Netherlands). Data were acquired on phantoms and in volunteers with the modified spoiled gradient-echo sequence using two subacquisitions with disjunct, uniform sampling in the phase encoding direction. Coil sensitivity and B0 maps were separately obtained, the latter by phase difference in phantoms and by chemical shift encoding-based water-fat separation in volunteers. Images were reconstructed from the two subacquisitions separately and jointly with the extended CS-PI reconstruction, optionally including a homodyne reconstruction. Phase errors were estimated from the individually produced images. Cerebral angiography was chosen for the first volunteer experiments, due to the importance of partial echo imaging in this application.
Results
A representative set of phantom images is provided in Figs. 3 and 4. A partial echo factor of 0.625 was simulated based on a full echo measurement with two interleaved subacquisitions with twofold acceleration in this case. Separately reconstructing the data from the two subacquisitions without a weighting primarily leads to ringing, as seen in Fig. 3 on the left. Evidently, synthesizing the missing data in readout direction, but not in phase encoding direction, poses a challenge to the CS-PI reconstruction without assuming smoothness of the phase. By contrast, jointly reconstructing all the data mainly results in aliasing, which is largely suppressed by integrating distortion and phase corrections into the reconstruction, as seen in Figs. 3 and 4 on the right. The reduced ringing suggests that accurately estimating the missing data succeeded.A selected set of time-of-flight angiograms is compiled in Fig. 5. A partial echo measurement with two interleaved subacquisitions without acceleration was performed in this case. Separately processing the data from the two subacquisitions with a smooth weighting as part of a homodyne reconstruction leads to a considerable difference in signal strength in particular in the internal carotid arteries. Apparently, the actual signal strength is once underestimated and once overestimated, depending on which half of the peripheral k-space in readout direction was actually measured. A consistent signal strength is obtained independent of the polarity of the readout gradient by jointly reconstructing half of the data from both subacquisitions.
Discussion
Reversing the polarity of the readout gradient during the acquisition enables sparsely sampling the full k-space with a Cartesian partial echo measurement, but it introduces inconsistencies between the two subacquisitions that must be considered in the reconstruction. The required distortion correction can also be based on a registration of the images generated individually from the two subacquisitions, as previously described for gradient-echo and echo-planar imaging, and the phase correction can be eliminated by a suitable calibration of the acquisition.4,5 Moreover, the masks $$$M_n$$$ need not be disjunct nor sample k-space uniformly in the phase encoding direction. Consequently, they may be optimized to facilitate these corrections. Interleaving the two subacquisitions, which is optional, can require a longer repetition time. Overall, the additional complexity incurred by bipolar partial echo imaging seems justifiable by the prospect of enhancing image quality.Acknowledgements
No acknowledgement found.References
1. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999; 42:952-962. 2. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing to rapid MR imaging. Magn Reson Med 2007; 58:1182-1195. 3. Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging 1991; 10:154-163. 4. Kannengiesser SAR, Wang Y, Haacke EM. Geometric distortion correction in gradient-echo imaging by use of dynamic time warping. Magn Reson Med 1999; 42:585-590. 5. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003; 20:870-888.Figures
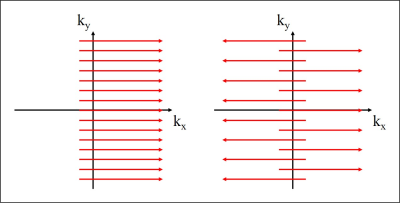
Fig. 1. k-space coverage in conventional unipolar
(left) and bipolar (right) partial echo imaging.
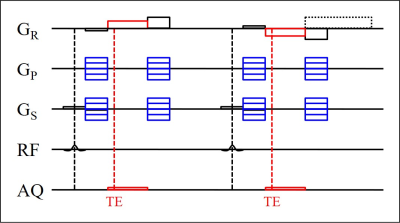
Fig. 2. Spoiled gradient-echo sequence for bipolar
partial echo imaging. The polarity of the gradients in readout direction (GR)
is reversed in the second shown repetition. The polarity of the trailing spoiler
gradient is optionally kept and the area is increased (dotted).
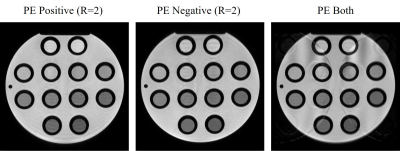
Fig. 3. Phantom images obtained from a bipolar partial
echo measurement, using the data acquired with positive polarity (left),
negative polarity (middle), and both polarities (right) of the readout gradient.
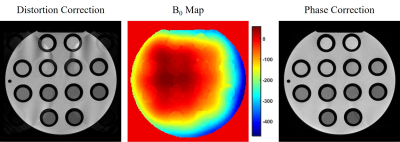
Fig. 4. Phantom images obtained from the same bipolar
partial echo measurement as in Fig. 3, using the data acquired with both polarities of the readout
gradient, with a distortion correction (left), based on a separately acquired B0
map (middle), and an additional phase correction (right).
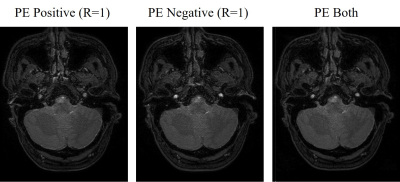
Fig. 5. Time-of-flight angiograms obtained from a bipolar
partial echo measurement, using the data acquired with positive polarity
(left) and negative polarity (middle) and half of the data acquired with each polarity (right) of the readout gradient.
DOI: https://doi.org/10.58530/2022/1715