1714
Simultaneous T2-weighted Real-time MRI of 2 Orthogonal Slices1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Division of Radiation Biophysics, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
In cancer radiotherapy, MR-guidance allows for superior tumor visualization and real-time target volume tracking. Real-time pulse sequences for MR-guidance require high acquisition speeds and good tumor-to-background contrast, which is often achieved with T2-weighted acquisitions. Here, we combine a real-time sequence with orthogonal slices with a T2w echo-shifted contrast and demonstrate its use under breathhold and free-breathing conditions in a volunteer.
Introduction
Real-time visualization of target volumes with enhanced accuracy can be achieved with MR-guidance during radiotherapy, focused ultrasound therapy (FUS), or laser-induced thermal therapy (LITT)1-3. MR sequences for these procedures require a high temporal resolution and the ability to resolve in- and out-of-plane motion. Conventional MR-guided radiotherapy procedures use fast T1-weighted (e.g. spoiled gradient echo, GRE), or T1/T2-weighted (e.g. balanced steady state free procession, bSSFP) sequences4,5. However, most applications require T2-weighted contrast to provide a clear delineation of the tumor from the healthy tissue1. In this work, we introduce a real-time sequence (PSIF-CROSS), which acquires two T2-weighted orthogonal slices simultaneously with a high temporal resolution.The PSIF-CROSS sequence combines a PSIF (time reversed FISP) sequence with crushed rephased orthogonal slice selection (CROSS)6. The PSIF sequence is an echo-shifted sequence where the spin echo (S-) is measured instead of the FID (S+), which generates a T2-weighted contrast with a high temporal resolution7. The CROSS acquisition scheme acquires two slices simultaneously, where a second orthogonal slice is excited between the excitation pulse and readout gradient6.
Methods
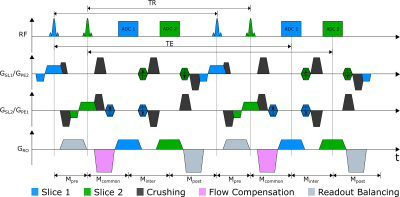
For rapid encoding the PSIF-CROSS sequence excites two orthogonal slices followed by two data acquisitions (Fig. 1). Both slices share the readout direction; however, phase encoding and slice selection directions are interchanged. To generate spin echoes for both slices in the subsequent TR, the 0th gradient moments M need to be balanced:$$-M_\text{pre}-M_\text{common}-M_\text{inter}-M_\text{post}+M_\text{pre}+M_\text{common}=0$$
$$-M_\text{common}-M_\text{inter}-M_\text{post}-M_\text{pre}+M_\text{common}+M_\text{inter}=0$$
which leads to
$$M_\text{pre}=-M_\text{post}=M_\text{inter}.$$
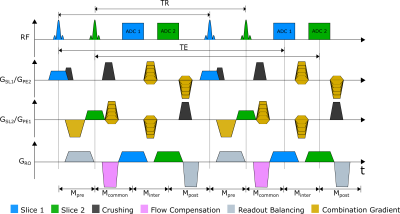
Interestingly, gradients in the common section do not contribute to the echo, and can thus be chosen arbitrarily. To dephase unwanted signal contributions from the orthogonal slice, crusher gradients were introduced. Additionally, the RF phase was alternated between ± 180 to shift signal contributions of the other slice by FOV/28. As the long echo times make the PSIF sensitive to motion artifacts, flow compensation was added to the sequence in the readout direction. Finally, gradients were combined to increase the acquisition speed (Fig. 2).
The PSIF-CROSS sequence was tested in in vivo under breathhold and free-breathing conditions in a 3T whole body MR system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a body array and spine array coil for signal reception. Images were acquired with the following parameters: α = 30°, TR/TE = 7.44/3.57 ms, BW = 1300 Hz/px, SL = 8 mm, FOV = 400×400 mm, matrix = 192, PF= 6/8, TA = 1.06 s. For comparison, sequential single slice images were acquired with a conventional PSIF sequence with the same parameters.
Results & Discussion
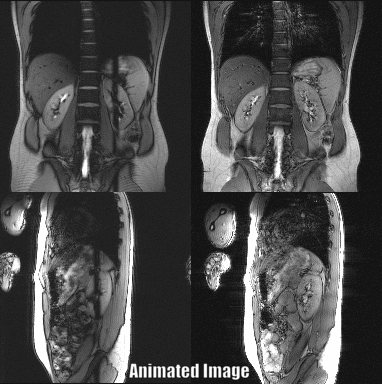
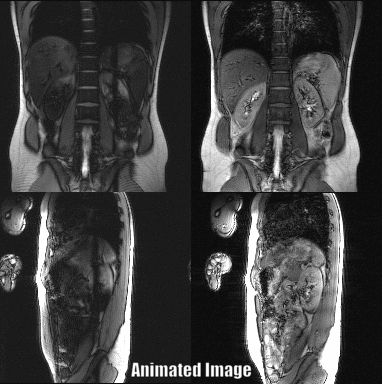
Abdominal images of a volunteer at the level of the kidney could be acquired with a temporal resolution of 1.06 s for both slices (Fig. 3). The renal motion due to breathing was successfully visualized using the PSIF-CROSS sequence (Fig. 4). Compared to the conventional PSIF sequence only minor qualitative differences such as increased dephasing is seen in the PSIF-CROSS sequence (Fig. 4).The PSIF-CROSS can acquire T2-weighted images with a sufficient temporal resolution for percutaneous interventions and slow breathing conditions, but the temporal resolution must be further increased (e.g., using parallel imaging) to use this sequence for tumor motion tracking in radiotherapy. The crusher gradients effectively eliminated signal interferences between the two slices, and the remaining artifacts in the overlap region were shifted by FOV/2 through RF phase cycling. Flow compensation in readout direction could partially compensate for motion, but some motion dephasing remained under free-breathing conditions - possibly a result of the crusher gradients used to separate signal. In future refinements of the sequence the crusher gradient amplitudes will be optimized.
Conclusion
The PSIF-CROSS sequence successfully acquires two T2-weighted orthogonal slices simultaneously with a higher temporal resolution than spin echo sequences. The sequence acquired orthogonal images in vivo under breathhold and free breathing conditions, and might be used in future work for tumor tracking in real-time in radiotherapy, FUS, or LITT. Further optimizing of the temporal resolution, flow compensation, crusher gradients, and saturation band region would increase robustness and suitability for MR-guidance applications.Acknowledgements
No acknowledgement found.References
1. Kurz C, et al. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol. 2020;15(93).
2. Carpentier A, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361-8.
3. Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417-430.
4. Tryggestad E, et al. 4D tumor centroid tracking using orthogonal 2D dynamic MRI: implications for radiotherapy planning. Med Phys. 2013;40(9):091712.
5. Mickevicius NJ, et al. Simultaneous orthogonal plane imaging. Magn Reson Med. 2017;78(5):1700-1710.
6. Krafft AJ, et al. Crushed rephased orthogonal slice selection (CROSS) for simultaneous acquisition of two orthogonal proton resonance frequency temperature maps. J. Magn. Reson. Imaging. 2013:38(6): 1510-1520
7. Moonen CT, et al. A fast gradient recalled MRI technique with increased sensitivity to dynamic susceptibility effects. Magn Reson Med 1992;26(1):184–189.
8. Tseytlin M. Concept of Phase Cycling in Pulsed Magnetic Resonance Using Sinusoidal Magnetic Field Modulation. Z Phys Chem (N F). 2017;231(3):689-703.
Figures