1695
Proton magnetic resonance spectroscopy of the distal metacarpus/tarsus in Thoroughbred racehorses with and without catastrophic fractures.1Royal Dick School of Veterinary Studies and Roslin Institute, Royal Dick School of Veterinary Studies and Roslin Institute, University of Edinburgh, Edinburgh, United Kingdom, 2BHF Centre for Cardiovascular Science and Edinburgh Imaging, BHF Centre for Cardiovascular Science and Edinburgh Imaging, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
Fractures of the third metacarpal/tarsal bone are common in racehorses. Racehorse cadaver limbs underwent single voxel magnetic resonance spectroscopy (MRS) and computed tomography. The percentage fat content (FC) and the mean bone mineral density (BMD) was calculated at 3 locations within the bone. A significant negative correlation was identified for mean BMD and percentage FC for all condyles and in bone marrow of the third metacarpal/tarsal distal diaphysis. The median percentage FC was lower in horses with fractures compared to controls. These findings suggest that fat and bone are capable of mutual regulation in Thoroughbred racehorses.
Introduction
Fractures of the third metacarpal or metatarsal bones (MC/MTIII) are common in racehorses1. Bone mineral density (BMD) and bone marrow adiposity are useful biomarkers in humans2,3. Peripheral quantitative computed tomography (pqCT) has been used to measure BMD in the MCIII of racehorses, showing changes in response to training4-6 but no differences have been identified between fractures and controls7. Magnetic resonance spectroscopy (MRS) allows quantification of marrow adipose tissue8,9 and previous studies in human bone have shown an inverse relationship between bone marrow adiposity and BMD3,10,11. Football players were found to have decreased bone marrow FC in the lumbar spine and femoral neck compared to controls12, and a similar finding was reported for runners13.The aim was to quantify the FC for the equine MC/MTIII and compare with CT-derived BMD. Our hypotheses were: 1) FC decreases with an increase in BMD in the trained racehorse, and 2) horses with fractures have a lower bone FC than horses without fractures.
Methods
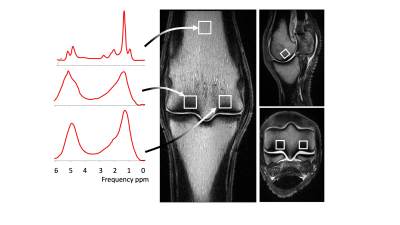
Limbs from Thoroughbred racehorses in training were recruited from racecourses between 2019 and 2021. Structural images were acquired at 3T (MAGNETOM Skyra; Siemens Healthineers, Erlangen, Germany) using a knee coil, including T1w TSE in 3 planes. Single voxel MRS was performed using a STEAM sequence (TE 20 ms, TR 4000 ms, 70 averages, 1cm3 voxel). Voxels were placed at 3 sites within the MC/MTIII, at the lateral and medial parasagittal grooves and in the centre of the distal diaphysis (proximal bone marrow). Spectra were analysed in JMRUI14 using AMARES15, fitting the water peak and 6 fat peaks (Fig.1). FC was calculated for each voxel as previously described3. CT scans were acquired on a helical 64-slice CT scanner (Somotom Definition AS; Siemens Healthineers, Erlangen, Germany) (120 kVp, 117 mAs, 0.4 mm slice thickness, 512x512 matrix size, 0.3 mm pixel spacing, table height 125 mm). Mean BMD was calculated for the lateral condyle, medial condyle and proximal bone marrow as previously described16.Analysis of the association between BMD and FC for each voxel location was performed using Pearson or Spearman correlations depending on normality. Kruskall-Wallis tests were used to check for associations between fracture groups and BMD or FC.
Results
Eighteen limbs from 10 horses with a median age of 9 years (range: 3-11 years) were included. Three limbs with metallic implants were excluded. One limb was excluded due to poor quality spectra. Limbs were divided into 2 groups; those from horses with clinical stress fractures (N=9) and those without (N=9). Fractured lateral condyles were excluded from the analysis (N=2). All condyles showed some sclerosis.The median value for mean BMD (IQR) was 830 (760-940) mg CaHAP/ml for the lateral condyle, 780 (720-860) mg CaHAP/ml for the medial condyle and 320 (280-350) mg CaHAP/ml for proximal bone marrow. The median FC (IQR) was 76 (70-83)% for the lateral condyle, 81 (67-83)% for the medial condyle and 90 (86-92)% for proximal bone marrow.
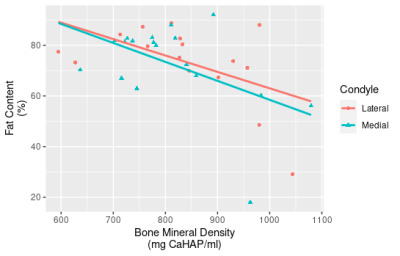
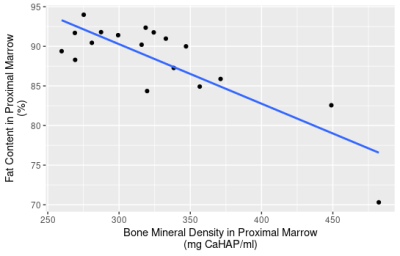
A significant negative correlation was identified between mean BMD and FC (correlation coefficient=-0.36; p=0.040) for all condyles (Fig.2) and for proximal bone marrow (correlation coefficient =-0.59; p=0.012) (Fig.3).
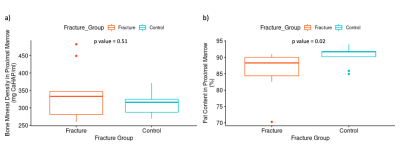
There was no significant difference between fracture and control groups for BMD or FC in either condyle (p=0.51, Fig. 4) but there was a significant difference between the median (IQR) FC in the proximal bone marrow (88 (84-90)%) for fracture versus control (91 (90-92)%)(p=0.02)(Fig.4).
Discussion
This is the first study to use MRS to quantify FC in equine bone. A significant negative correlation was identified between BMD and FC of bone in the condyles and bone marrow of the distal diaphysis of MC/MTIII thereby supporting our hypothesis. Horses with stress fractures were found to have a significantly lower marrow FC in the distal diaphysis compared to controls. A similar association was not found for the FC within the condyles, likely due to the presence of the sclerotic bone occupying the distal condyles of racehorses in training. Previous studies have found fat and bone to be capable of mutual regulation in humans17-19. In racehorses, the negative correlation in the MC/MTIII condyles can be explained by adaptive modelling in response to load during exercise20.Previous studies have identified an increase in BMD in the condyles of MTIII in trained racehorses compared to untrained racehorses20-22. Furthermore, racehorses that sustain fractures of MCIII have been found to have an increased bone volume fraction and less porous subchondral bone at the fracture site using high resolution pqCT23. The adaptive modelling process within the subchondral bone of the distal MC condyle leads to bone deposition on the surface of the trabelculae, reduction in the size of the fat-filled marrow spaces and a reduction in bone marrow adiposity20. Although a decrease in marrow FC has been reported in human athletes compared to controls, the exact mechanism for this is not fully understood12.
Conclusion
This study shows that 1H MRS can be performed in the equine MC/MTIII and that FC decreases with an increase in BMD in the trained racehorse in the condyles and proximal marrow. Horses with fractures have a lower FC in the proximal marrow than horses without fractures suggesting that fat and bone are capable of mutual regulation in Thoroughbred racehorses.Acknowledgements
We would like to acknowledge the Horseracing Betting Levy Board for their generous funding of Charlotte Hewitt-Dedman’s Clinical Scholarship.References
1. Allen SE, Rosanowski SM, Stirk AJ, Verheyen KLP. Description of veterinary events and risk factors for fatality in National Hunt flat racing Thoroughbreds in Great Britain (2000-2013). Equine Vet J. Nov 2017;49(6):700-705.
2. Zhao Y, Huang M, Ding J, et al. Prediction of Abnormal Bone Density and Osteoporosis From Lumbar Spine MR Using Modified Dixon Quant in 257 Subjects With Quantitative Computed Tomography as Reference. Journal of magnetic resonance imaging. 2019;49(2):390-399.
3. Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. Aug 2013;28(8):1721-8.
4. Bogers SH, Rogers CW, Bolwell C, Roe W, Gee E, McIlwraith CW. Quantitative comparison of bone mineral density characteristics of the distal epiphysis of third metacarpal bones from Thoroughbred racehorses with or without condylar fracture. Am J Vet Res. Jan 2016;77(1):32-8.
5. Firth EC, Rogers CW, Doube M, Jopson NB. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 6. Bone parameters in the third metacarpal and third metatarsal bones. New Zealand veterinary journal. 2005;53(2):101-112.
6. Firth EC, Rogers CW, Rene van Weeren P, et al. The effect of previous conditioning exercise on diaphyseal and metaphyseal bone to imposition and withdrawal of training in young Thoroughbred horses. The veterinary journal (1997). 2012;192(1):34-40.
7. Loughridge AB, Hess AM, Parkin TD, Kawcak CE. Qualitative assessment of bone density at the distal articulating surface of the third metacarpal in Thoroughbred racehorses with and without condylar fracture. Equine Veterinary Journal. 2017/03/01 2017;49(2):172-177.
8. Seifert AC, Wehrli FW. Solid-State Quantitative (1)H and (31)P MRI of Cortical Bone in Humans. Curr Osteoporos Rep. Jun 2016;14(3):77-86.
9. Xu K, Sigurdsson S, Gudnason V, Hue T, Schwartz A, Li X. Reliable quantification of marrow fat content and unsaturation level using in vivo MR spectroscopy: Quantifying Marrow Fat and Unsaturation Level With In Vivo MRS. Magnetic resonance in medicine. 2018;79(3):1722-1729.
10. Yeung DKW, Griffith JF, Antonio GE, Lee FKH, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. Journal of magnetic resonance imaging. 2005;22(2):279-285.
11. Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? Journal of magnetic resonance imaging. 2012;35(1):117-124.
12. Wang J, Yi P, Huang Y, et al. Quantitative evaluation of bone marrow fat content and unsaturated fatty index in young male soccer players using proton magnetic resonance spectroscopy (1H-MRS): a preliminary study. Quantitative imaging in medicine and surgery. 2021;11(10):4275-4286.
13. Belavy DL, Quittner MJ, Ridgers ND, Shiekh A, Rantalainen T, Trudel G. Specific Modulation of Vertebral Marrow Adipose Tissue by Physical Activity. Journal of bone and mineral research. 2018;33(4):651-657.
14. Naressi A, Couturier C Fau - Devos JM, Devos Jm Fau - Janssen M, et al. Java-based graphical user interface for the MRUI quantitation package. Magn Reson Mater Phy. 2001;12:141-152.
15. Karampinos DC, Melkus G, Baum T, Bauer JS, Rummeny EJ, Krug R. Bone marrow fat quantification in the presence of trabecular bone: initial comparison between water-fat imaging and single-voxel MRS. Magn Reson Med. Mar 2014;71(3):1158-65.
16. Woods G, Israeliantz Gunz NA-OX, Handel I, Liuti TA-O, Mellanby RA-O, Schwarz TA-OX. Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head-Preliminary Results. Animals. 2021;11(5):1413-1425.
17. Warriner AH, Mugavero MJ. Bone Changes and Fracture Risk in Individuals Infected With HIV. Current Rheumatology Reports. 2010/06/01 2010;12(3):163-169.
18. Buehring B, Kirchner E, Sun Z, Calabrese L. The Frequency of Low Muscle Mass and Its Overlap With Low Bone Mineral Density and Lipodystrophy in Individuals With HIV—A Pilot Study Using DXA Total Body Composition Analysis. Journal of clinical densitometry. 2012;15(2):224-232.
19. Rigotti NA, Nussbaum SR, Herzog DB, Neer RM. Osteoporosis in Women with Anorexia Nervosa. The New England journal of medicine. 1984;311(25):1601-1606.
20. Boyde A, Firth EC. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 8. Quantitative back-scattered electron scanning electron microscopy and confocal fluorescence microscopy of the epiphysis of the third metacarpal bone. New Zealand Veterinary Journal. 2005/04/01 2005;53(2):123-132.
21. Riggs CM, Boyde A. Effect of exercise on bone density in distal regions of the equine third metacarpal bone in 2-year-old thoroughbreds. Equine Vet J Suppl. Jul 1999;(30):555-60.
22. Bogers SH, Rogers CW, Bolwell CF, Roe WD, Gee EK, McIlwraith CW. Impact of race training on volumetric bone mineral density and its spatial distribution in the distal epiphysis of the third metatarsal bone of 2-year-old horses. The veterinary journal (1997). 2014;201(3):353-358.
23. Whitton RC, Trope Gd Fau - Ghasem-Zadeh A, Ghasem-Zadeh A Fau - Anderson GA, et al. Third metacarpal condylar fatigue fractures in equine athletes occur within previously modelled subchondral bone. 2010;47:826-831.
Figures