1693
Comparison of CT versus Zero Echo Time MRI techniques in the management of patients with shoulder instability1GE Healthcare, Madrid, Spain, 2Rey Juan Carlos University, Madrid, Spain, 3Clínica Cemtro, Madrid, Spain, 4GE Healthcare, San Diego, CA, United States, 5GE Healthcare, Waukesha, WI, United States, 6GE Healthcare, New York, NY, United States
Synopsis
In this study we evaluate the viability of using ZTE, a novel MRI sequence for bone imaging, with deep-learning (DL) reconstruction to assess glenohumeral shoulder instability, aiming to improve signal-to-noise ratio (SNR) and get comparable images to CT, the gold standard technique for surgical planning. Bone loss measurements were performed on both techniques achieving almost perfect inter-modality agreement on 20 patients. This approach could prevent the patient from receiving ionizing radiation concomitant to CT examination and could be combined in a single routine shoulder examination with other MR sequences for a complete study and optimized patient workflow.
Background
Glenohumeral instability is a common problem following traumatic anterior shoulder dislocation, which corresponds to the most dislocated joint1. Two major risk factors of recurrent instability are glenoid and Hill-Sachs bone loss2. Recently, consideration of the interactions of these types of bipolar bone loss has been used to determine if a lesion is ‘on-track’ or ‘off-track’ and select the appropriate surgical technique (arthroscopic repair, coracoid or bone grafting),3,4,5. Successful outcomes can be compromised by failure to quantify bone loss and assess risk of engagement6,7.Although 3D Computed Tomography (CT) is the gold standard for assessment and presurgical planning, due to the high correlation with arthroscopic evaluation8,9, 3D Zero echo time (ZTE) technique has been recently used to image the osseous structures of the shoulder10,11. ZTE is a valuable diagnostic tool in osteoarticular Magnetic Resonance (MR) since it acquires signal from short T2 tissues and can depict bone surface unlike other MR techniques. ZTE could be a promising alternative to CT in this scenario, avoiding radiation and managing patients in a single MR examination. However, the standard ZTE techniques are prone to low signal-to-noise ratio (SNR) and resolution.
Recently the use of deep learning (DL) reconstruction methods has become available in the MR environment to improve image quality and reduce acquisition times. The application of these DL methods to the standard ZTE techniques could make them feasible in the assessment of the glenohumeral joint.
In this work we explore this possibility comparing the DL ZTE images against the routine CT examination.
Subject Population
20 patients with glenohumeral instability were recruited in Clinica Cemtro, Madrid (Spain) for this study, prior approval from Ethics Committee.Data Acquisition Methods
All patients underwent CT and MRI exams on the same day. CT scanning was performed on a 256-slice scanner (Revolution CT, GE Healthcare, Milwaukee, WI) with the following scan parameters: slice thickness, 1.25 mm; reconstruction matrix, 512; field of view, 20 to 30 cm; 140 kV (peak); and pitch factor 0.98.MRI was conducted on a 3T SIGNA™ Architect (GE Healthcare, Waukesha, WI) scanner with a 20-channel AIRTM multi-purpose coil (GE Healthcare, Waukesha, WI), adding to the routine shoulder clinical protocol a 3D radial ZTE sequence12; flip angle = 1° , repetition time (TR)= 88.6ms, field of view (FOV)=18cm, resolution = 1x1x1mm, bandwidth =62.5kHz, number of excitations (NEX)=4, acquisition time=~2 minutes.
The DL reconstruction method employed to improve SNR of ZTE MRI uses a deep convolutional residual encoder network trained to reconstruct images with minimal noise13 (tuned to 75% denoising for this work). A bias-correction algorithm was applied to correct for signal inhomogeneities due to coil geometry. Then signal intensity was inverted to provide CT-like contrast (Figure 1).
Data Analysis Methods
When identifying bipolar bone lesions at risk of engagement, glenoid track (GT), the contact area between the humeral head and glenoid during shoulder abduction and external rotation, becomes essential along with the Hill–Sachs interval (HSI), that includes the width of the Hill–Sachs lesion (HSL) plus the width of the intact bone bridge (BB)3,14.One radiologist with 5 years of experience performed the required measurements (Figure 2) to categorize each patient as on-track/off-track on a GE AW workstation (GE Healthcare, Waukesha, WI).
GT was quantified on a reformatted sagittal ‘en face’ view of the glenoid from both ZTE and CT volumes independently (Figure 3 (A, B)). HSI was measured on the rendered 3D volumes (Figure 3 (C, D)).
Statistical analyses were performed using the SPSS software package (version 22; IBM, Armonk, NY). Intraclass correlation coefficient (ICC) values were calculated to assess intermodal agreement on measuring GT and HSI. Bias and limits of agreement values were calculated with the methods of Bland and Altman. Additionally, Cohen’s Kappa agreement values were calculated between techniques as to whether the shoulder was on-track or off-track.
Results
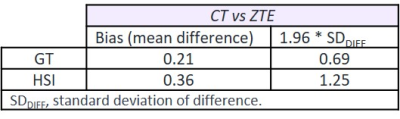
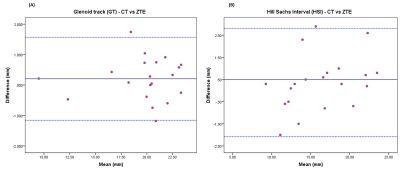
Readers found no critical issues when measuring on both techniques. Thanks to the information from routine MRI exam, bone bridge could be better assessed on ZTE than on CT.ICCs (95% CI, p < 0.001) for inter-modality assessment showed almost perfect agreement for both measurements: GT - 0.99 (0.974, 0.996) and HSI - 0.978 (0.945,0.991). Based on the Bland-Altman analysis (Table 1, Figure 4), no differences in bias were noted for the measurements between both techniques.
On-track/off-track classification demonstrated good agreement for both techniques based on kappa statistics (kappa = 0.765, p<0.001). Nevertheless, 100% agreement was not achieved since two cases were off-track in CT and on-track in ZTE, due to small differences between GT and HSL (<0.5mm). These critical cases would be considered along with all the clinical information to take the treatment decision.
Conclusion
Thanks to the addition of the novel DL reconstruction method, ZTE provided comparable CT image quality showing high inter-modality agreement and making it an excellent tool for surgical planning. This approach will save the patient from receiving ionizing radiation concomitant to CT examination and could be combined in a single exam with routine shoulder MR sequences optimizing patient workflow.As a future work, patient outcomes could be compared with the treatment path suggested by the obtained measurements and on/off-track classification within this study, to assess its predictive prognostic power.
Acknowledgements
1. Nabian MH, Zadegan SA, Zanjani LO, Mehrpour SR. Epidemiology of Joint Dislocations and Ligamentous/Tendinous Injuries among 2,700 Patients: Five-year Trend of a Tertiary Center in Iran. Arch Bone Jt Surg. 2017;5(6):426-434.
2. Saliken D, Bornes T, Bouliane M, Sheps D, Beaupre L. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review.BMC Musculoskelet Disord. 2015;16(1). doi:10.1186/s12891-015-0607-1
3. Di Giacomo G, Itoi E, Burkhart S. Evolving Concept of Bipolar Bone Loss and the Hill-Sachs Lesion: From “Engaging/Non-Engaging” Lesion to “On-Track/Off-Track” Lesion. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2014;30(1):90-98. doi:10.1016/j.arthro.2013.10.004
4. Locher J, Wilken F, Beitzel K, et al. Hill-Sachs Off-track lesions as risk factor for recurrence of instability after arthroscopic Bankart repair. Arthroscopy 2016;32:1993-1999.
5. Shaha JS, Cook JB, Rowles DJ, et al. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg [Am] 2016;98:1918-1923.
6. Randelli P, Ragone V, Carminati S, Cabitza P. Risk factors for recurrence after bankart repair a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20:2129–38.
7. Ho AG, Gowda AL, Michael Wiater J. Evaluation and treatment of failed shoulder instability procedures. Journal of Orthopaedics and Traumatology. 2016;17(3):187-197. doi:10.1007/s10195-016-0409-8
8. Bishop J, Jones G, Rerko M, Donaldson C. 3-D CT is the Most Reliable Imaging Modality When Quantifying Glenoid Bone Loss. Clinical Orthopaedics & Related Research. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x
9. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy 2008;24:376-382.
10. Breighner RE, Endo Y, Konin GP, Gulotta LV, Koff MF,Potter HG. Technical developments: Zero echo time imaging of the shoulder: Enhanced osseous detail by using MR imaging. Radiology 2018;286:960-966.
11. De Mello R, Ma Y, Ashir A et al. Three-Dimensional Zero Echo Time Magnetic Resonance Imaging Versus 3-Dimensional Computed Tomography for Glenoid Bone Assessment. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2020;36(9):2391-2400. doi:10.1016/j.arthro.2020.05.042
12. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, et al. Zero TE MR bone imaging in the head: Zero TE Bone Imaging. Magn Reson Med. 2015 Jan;75(1):107–14.
13. Lebel R. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. https://arxiv.org/abs/2008.06559. Published 2020. Accessed Nov 2021.
14. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-656.
15. Huijsmans PE, Haen PS, Kidd M, Dhert WJ, van der Hulst VP, Willems WJ. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: a cadaveric study. J Shoulder Elbow Surg. 2007 Nov-Dec;16(6):803-9.
16. Gyftopoulos S, Hasan S, Bencardino J, Mayo J, Nayyar S, Babb J, Jazrawi L. Diagnostic accuracy of MRI in the measurement of glenoid bone loss. AJR Am J Roentgenol. 2012 Oct;199(4):873-8
17. Omori Y, Yamamoto N, Koishi H, et al. Measurement of the glenoid track in vivo as investigated by 3-dimensional motion analysis using open MRI. Am J Sports Med 2014;42:1290-1295
References
No reference found.Figures
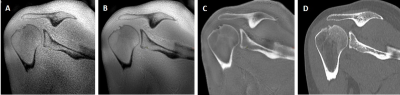
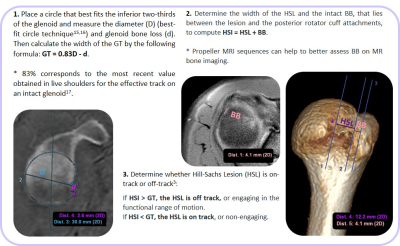
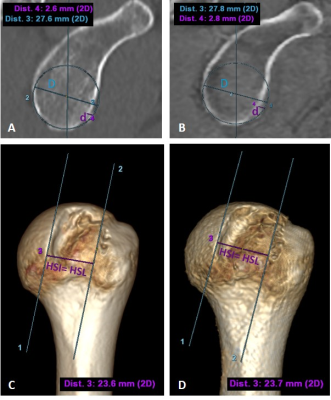
Figure 3. GT measurement through best-fit circle on a sagittal ‘en-face’ glenoid view - (A) CT and (B) bone image from ZTE. Both diameter (D) and glenoid bone loss (d) are shown in blue and purple respectively. Leading to a GT of 20.31mm for CT and 20.27mm for ZTE. HSI is measured on 3D volumes (C) CT, (D) ZTE. In this case, there is no BB so all the HSI corresponds to the HSL, 23.6mm for CT and 23.7mm for ZTE. Since HSI > GT on both techniques, this HSL was classified as off-track.