1690
23Na in vivo MRI with 1mm isotropic resolution and real-time prospective motion correction implemented in Pulseq1High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 2High Field MR Center, Department of Biomedical Imaging and Image- Guided Therapy, Medical University of Vienna, Vienna, Austria
Synopsis
Sodium imaging has a great potential for basic research and clinical MRI, but its applications are limited due to poor SNR achievable in typical in vivo scanning times. Image acquisition times can be substantially extended if involuntarily subject motion is compensated for, e.g. by using an external optical motion tracking system. In this proof-of-concept study we demonstrate 1mm isotropic 23Na MRI with the achieved image quality sufficient for the anatomical orientation based on the native sodium images. The used ultra-short echo time 3D imaging sequence was implemented in Pulseq, an open-source platform-independent development environment.
Introduction
The direct correlation of sodium concentration with the glycosaminoglycan content, which reflects the biochemical and biomechanical properties of musculoskeletal tissues such as cartilage and tendons, makes the application of sodium imaging highly attractive [1,2]. The earliest stages of cartilage degeneration are associated with a loss of glycosaminoglycans [3]. In joints, cartilage layer can be visualized by sodium imaging with a unique contrast to the surrounding tissues. However, in vivo 23Na MRI applications remain hampered by the low SNR, severely constraining the achievable resolution. Ultra-high field scanners with the higher SNR allow for improved X-nuclei imaging [2], but even at 7T the spatial resolution of sodium imaging remains at the level of 2 mm in-plane, often with thicker slices or slabs [1,2].Prospective motion correction (PMC) is becoming a true enabler for high-resolution neuroimaging in vivo by allowing for substantially longer imaging times [4,5]. PMC eliminates the widely accepted limitation of 10 to 15 minutes in terms of the maximum acquisition duration of a single data set, allowing for continuous scanning and signal averaging over several hours [6]. PMC has also shown its ability to compensate for unavoidable motion under mechanical loading in functional knee imaging [7,8].
In the present study we aim at high-resolution three-dimensional 23Na MRI of the knee joint with isotropic spatial resolution of 1mm. In vivo imaging was done with a tailored pulse sequence inspired by PETRA and WASPI techniques [9,10], optimized for very short signal life times of sodium and implemented in Pulseq [11]. We compensate for the lack of SNR by extending the imaging time to ~1 hour, whereas PMC based on the optical motion tracking [12] is used to correct for involuntarily motion.
Methods
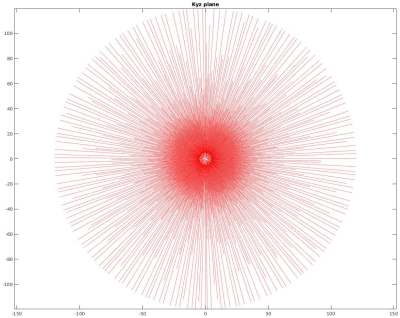
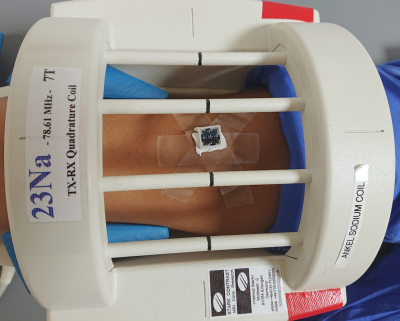
Imaging sequence has been developed in Pulseq [11] using Matlab (Mathworks, Natick, MA, USA). The readout gradient was ramped up and the spin excitation was achieved by a 50us-short RF pulse with a flip angle of 6°. Following a TX/RX switching delay of 60us ADC was activated to acquire a radial center-out k-space spoke of 240 data points with a dwell time of 6us. Thereafter, the direction of the gradient was altered without changing its magnitude and the TR period was repeated. The end points of the spokes were set to cover homogeneously a sphere in k-space with the diameter corresponding to the nominal resolution of 1mm and the undersampling factor of 8 (22620 spokes). Gradient directions were following the phyllotaxis scheme with the interleaving selected to minimize the average step distance between the neighboring TRs. For filling out the missing samples at the k-space center the gradient amplitude was scaled down and the same phyllotaxis algorithm was used to calculate the sampling directions, but this time to fulfill Nyquist sampling for the FOV of 240mm on the inner k-space sphere. Only 32 initial ADC samples were acquired for the inner spheres with the total of 5 spheres, followed by a TR without gradients, resulting in 696 additional TRs. Fig. 1 shows a thin slab through the sampled k-space trajectory. The entire sequence was repeated 48 times for averaging, leading to the total measurement duration of 56:24.Imaging was done on Siemens 7T Dot+ MR system (Siemens Healthineers, Erlangen, Germany). PMC of the exported Pulseq sequence was achieved as described previously [13]. In short: libXPACE, a library for the eXternal Prospective Acquisition CorrEction [12], was linked to the customized Pulseq interpreter sequence and updated orientation and position information of the pulse sequence blocks; appropriate temporal position for such updates were either detected automatically or optionally based on the specialized “labels” in the Pulseq sequence. An external optical motion tracking system (Metria Innovation, Milwaukee, WI, USA) was used to track the motion of the marker in 6 degrees-of-freedom at 85Hz. The marker was attached to the knee cap of a volunteer (see Fig 2) positioned in a 23Na birdcage coil (Stark Contrast, Erlangen Germany). This single-channel birdcage coil was used to allow for the tracking camera to view the marker. Acquired raw data were streamed to an external PC together with the used Pulseq sequences and images were reconstructed off-line using PICS algorithm with L1-regularization implemented in the BART software package [14].
Results and Discussion
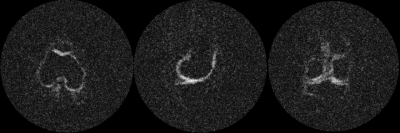
The volunteer remained very still in both motion corrected and non-corrected scans with a comparable standard deviations of position of ~1mm. Fig. 3 demonstrates the achieved image quality for 1mm isotropic sodium imaging. Fig. 4 visualizes consecutive slices from the same data set, demonstrating the richness of the acquired information. Motion corrected images were consistently sharper than non-corrected scan (data not shown).This proof-of-concept study demonstrates the feasibility of 1mm isotropic sodium imaging in vivo. Image SNR can be further improved by utilizing tighter-fitting array coils tailored for combination with optical motion tracking. Further benefits are expected from a combination of the presented acquisition with the AI-powered image reconstruction [15].
Conclusions
This study demonstrates the feasibility of 1mm isotropic sodium imaging in vivo and motivates further RF coil, pulse sequence and image reconstruction development.Acknowledgements
Patrick Hucker from the University Medical Center Freiburg, Freiburg, Germany is acknowledged for developing and maintaining the libXPACE software.References
1 Zaric O et al. Frontiers of Sodium MRI Revisited: From Cartilage to Brain Imaging. J. Magn. Reson. Imaging 2021;54:58–75, doi: 10.1002/jmri.27326
2 Aringhieri G, et al. Musculoskeletal MRI at 7 T: do we need more or is it more than enough? Eur Radiol Exp 2020; 4, 48, doi:10.1186/s41747-020-00174-1
3 Zbýň S, et al. Assessment of Low-Grade Focal Cartilage Lesions in the Knee With Sodium MRI at 7 T: Reproducibility and Short-Term, 6-Month Follow-up Data. Invest Radiol 2020; 55(7):430-437, doi: 10.1097/RLI.0000000000000652.
4 Stucht D, et al. Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction, PLoS One 2015; 10(7):e0133921, doi:10.1371/journal.pone.0133921
5 Mattern H, et al. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magn Reson Med 2018; 80:248-258, doi: 10.1002/mrm.27033
6 Lüsebrink F, et al. Comprehensive ultrahigh resolution whole brain in vivo MRI dataset as a human phantom. Sci Data. 2021; 8: 138, doi: 10.1038/s41597-021-00923-w
7 Lange T, et al. Knee cartilage MRI with in situ mechanical loading using prospective motion correction. Magn Reson Med. 2014; 71:516-23. doi: 10.1002/mrm.24679
8 Lange T, et al. Comparative T2 and T1rho mapping of patellofemoral cartilage under in situ mechanical loading with prospective motion correction. J Magn Reson Imaging. 2017;46:452-460. doi: 10.1002/jmri.25574
9 Grodzki DM, et al. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 2012; 67:510-8, doi: 10.1002/mrm.23017
10 Wu Y, et al. Water- and fat-suppressed proton projection MRI (WASPI) of rat femur bone. Magn Reson Med 2007; 57:554–567, doi: 10.1002/mrm.21174
11 http://pulseq.github.io/
12 Maclaren J, et al. 2012 Measurement and Correction of Microscopic Head Motion during Magnetic Resonance Imaging of the Brain. PLoS ONE 7(11): e48088. doi: 10.1371/journal.pone.0048088
13 Zaitsev M, et al. Real-time prospective motion correction of arbitrary MR pulse sequences with XPACE-Pulseq. ISMRM 2021, #1378
14 http://mrirecon.github.io/bart/
15 Hammernik K, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med 2018;79:3055-3071. doi: 10.1002/mrm.26977
Figures