1687
Minimum number of scans for magic angle directional imaging based on a priori anatomical information
Harry Lanz1, Karyn Elizabeth Chappell2, and Mihailo Ristic3
1Mechanical Engineering, Imperial College London, London, United Kingdom, 2Surgery and Cancer, Imperial College London, London, United Kingdom, 3Mechanical Engineering Department, Imperial College London, London, United Kingdom
1Mechanical Engineering, Imperial College London, London, United Kingdom, 2Surgery and Cancer, Imperial College London, London, United Kingdom, 3Mechanical Engineering Department, Imperial College London, London, United Kingdom
Synopsis
By exploiting the field-dependent anisotropy of collagen, valuable clinical information can be obtained, such as fibre tractography. We investigated the use of a priori anatomical knowledge such as scout scan, using both simulations and experimentally acquired images. We conclude that only 3 scanning directions may be sufficient for robust analysis, instead of 9 or more in the case when no such information is used. The method is also compatible with low SNR values associated with low-field MRI.
Introduction
In collagen-rich tissues, MR image intensity depends on the angle, θ, between B0 and the collagen fibres, reaching a maximum at the “magic angle” 1, θ = 55°. Magic Angle Directional Imaging (MADI)2 involves imaging at different angles θ and analysing the intensity changes to determine the dominant collagen orientation in each voxel. Original work1 suggested that 15 orientations may be needed, while subsequent research showed 9 or fewer orientations may be sufficient to estimate arbitrary fibre directions, depending on the image SNR3.Here, we explore whether prior knowledge of the target anatomy can be used to further reduce the required number of scanning directions. We hypothesised that if an approximate fibre direction is known a priori, then only 3 scanning directions may be sufficient for its accurate estimation. For example, the dominant fibre orientation in healthy tissue may be expected to follow the path of the particular ligament or tendon being studied, and this may be determined from a preceding scout scan. With practical applications in mind, we also employed soft registration of volume images based on mutual information, instead of previously proposed rigid-body registration using fiducial markers.
Methods
MADI methodA total of N MR images must first be acquired at different angles, θ, of B0 relative to the subject. Volume MR images were parameterised using B-splines to accommodate any geometric distortion due to scanner characteristics and registered by soft registration4 with mutual information5 as the optimisation metric, implemented using Elastix [6]. A user defined Region of Interest (ROI) is selected, and voxels showing intensity variation with standard deviation above a threshold were detected. Image intensity I as a function of the field angle θ can be modelled as1:
I=A exp(-B (3cos2θ-1)2 ) (1)
where A and B are constants. This expression was used to simulate intensities, IC , for a bouquet of predefined test directions, equally spread over a hemisphere. Correlation of the measured intensities, IM , for each voxel with the simulated ones was used to find an initial guess for the subsequent simplex minimisation, which computes the estimate of the actual fibre direction.
Simulation study
A single collagen-containing voxel of known orientation was simulated using Equation 1 with added noise. Considering various possible combinations of 3 scanning directions in 3D, we simulated fibre estimation for corresponding θ1, θ2, θ3 in the range 15° − 85°. The deviation of the computed fibre direction from the ground truth was recorded.
MR image study
15 volume images of a caprine knee specimen were obtained at orientations equally spaced over a hemisphere, allowing for any collagen direction in the scans to be computed. Fibre directions obtained in this way served as a gold standard for subsequent comparisons. We then used a subset of 3 of these scans, choosing one that was closest to θ = 55° relative to the patella tendon, while the other two were at θ values approximating the optimal ones found in the simulation studies. This data set also had Gaussian noise applied incrementally to study the effect of reduced SNRs.
Results
Figure 1 shows the simulation results as the deviation of the computed fibre direction from ground truth, for 15° < θ1,2 < 85° and θ3 = 55°. There is a clear region of minimum error when one of θ1,2 is < 55° and the other is > 55°.Figure 2 shows the final tractography plots of the caprine patella tendon. Figure 2a was obtained using all 15 equally spaced scanning directions. Figure 2b shows the results using only a subset of 3 scanning directions, with scans corresponding to angles of 38°, 56° and 64° between B0 and the estimated orientation of the patella tendon.
Figure 3 analyses the robustness of the 3-scan fibre estimation in relation to image SNR, showing the proportion of the fibre orientation estimates being within 25° of the 15-scan gold standard.
Discussion
Fibre directions estimation was performed using correlation, and it could be expected that this is best achieved if the measurements capture the shape of the curve I vs. θ, which has a peak at θ ≈ 55°. The resulting errors may be expected to be small if the 3 directions involve θ both above and below 55°. Using < 3 scans does not lend itself to achieving accurate results and simulation studies confirm this.The method was found to remain robust for low SNR values, which may be encountered using low-field MRI.
In diagnostics, scout scans may be used to establish expected fibre orientation of healthy tissue and MADI may establish what proportion of the tissues remains healthy. Minimimising the number of scanning directions directly reduces the overall scanning time.
Conclusions
Use of a priori information can enable using only 3 scans to achieve successful collagen tractography. Robustness at low SNR means the method is applicable with low field scanners. In the next stage of our research we plan to conduct in vivo studies using our prototype permanent magnet scanner7 which can provide arbitrary orientation of B0.Acknowledgements
No acknowledgement found.References
- Nikolaus M. Szeverenyi and Graeme M. Bydder. Dipolar anisotropy fiber imaging in a goat knee meniscus. Magnetic Resonance in Medicine, 65(2):463–470, 2011.
- Karyn E. Chappell, Djordje Brujic, Catherine Van Der Straeten, Richard Meeson, Wladyslaw Gedroyc, Donald McRobbie, and Mihailo Ristic. Detection of maturity and ligament injury using magic angle directional imaging. Magnetic Resonance in Medicine, 82(3):1041–1054, 2019.
- Djordje Brujic, Karyn E. Chappell, and Mihailo Ristic. Accuracy of collagen fibre estimation under noise using directional mr imaging. Computerized Medical Imaging and Graphics, 86:101796, 2020.
- D Rueckert, L.I Sonoda, C Hayes, D.L.G Hill, M.O Leach, and D.J Hawkes. Nonrigid registration using free- form deformations: application to breast mr images. IEEE transactions on medical imaging, 18(8):712–721, 1999.
- F Maes, A Collignon, D Vandermeulen, G Marchal, and P Suetens. Multimodality image registration by maximization of mutual information. IEEE transactions on medical imaging, 16(2):187–198, 1997.
- S. Klein, M. Staring, K. Murphy, M. A. Viergever, and J. P. W. Pluim. elastix: A toolbox for intensity-based medical image registration. IEEE Transactions on Medical Imaging, 29(1):196–205, 2010.
- John V.M. McGinley, Mihailo Ristic, and Ian R. Young. A permanent mri magnet for magic angle imaging having its field parallel to the poles. Journal of Magnetic Resonance, 271:60 – 67, 2016.
Figures
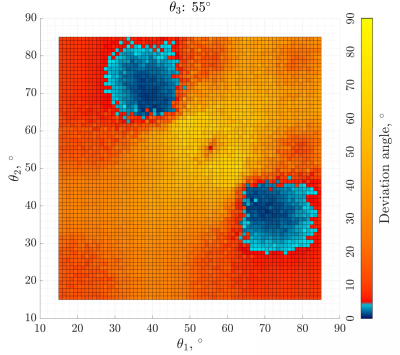
Figure
1: Simulation results showing deviation of the estimated fibre orientation from
ground truth obtained using 3 scanning directions corresponding to 15°<θ1,2 <85° and θ3=55°.
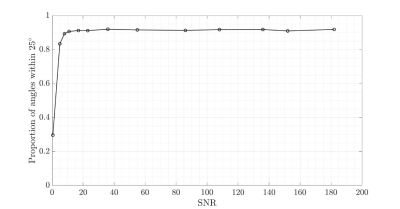
Figure
2: Simulation results showing the fraction of calculated collagen fibre
orientations within 25° of the ground
truth for different image signal-to-noise ratios.
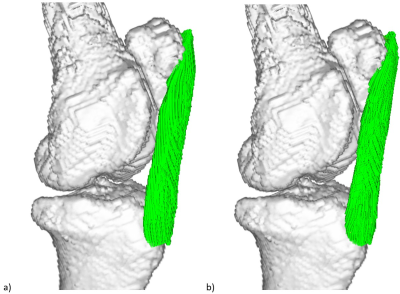
Figure 3: Tractography plots of a caprine patella tendon
with superimposed bone structure, a) using 15 scan directions equally
distributed on a hemisphere and no a priori assumption about the dominant fibre
orientation; b) using a subset of 3 of the 15 scans corresponding to θ1,2,3=38°,56°,64°
between B0 and the estimated orientation of the patella
tendon.
DOI: https://doi.org/10.58530/2022/1687