1681
Breast DTI: A Prospective Study in Individuals Referred for Biopsy of Indeterminate Breast Lesions1NorthShore University HealthSystem, Evanston, IL, United States, 2Radiology, NorthShore University HealthSystem, Evanston, IL, United States
Synopsis
We present preliminary data from an ongoing study in individuals referred for biopsy of BIRADS 3, 4, and 5 breast lesions based on mammography, ultrasound, and/or screening MRI. DTI data was acquired using single shot EPI with 30 different directions and 2 mm isotropic resolution. MRI data was analyzed using BIT-Motion software. All cancers showed low ADC and λ1 values compared to control regions. FA, consistent with prior reports showed minimal difference and so did (λ1-λ3).
INTRODUCTION
While Breast Diffusion MRI has shown promise in differentiating malignant from benign and control tissue [1], the experience to-date with DTI is still evolving. Retrospective studies have shown promise of primary Eigen value λ1, ADC and (λ1-λ3) [2, 3]. All studies to-date have been performed as an add-on to clinical studies involving DCE-MRI. The high negative biopsy rate of breast DCE MRI restricts its use for evaluating indeterminate breast lesions [4].Here, we present preliminary data from a single-site prospective study in individuals with suspicious breast lesions that were referred to biopsy. The goal of this study was to determine whether non-contrast MRI with DTI acquired prior to biopsy can reliably identify benign lesions that do not require biopsy.
MATERIALS & METHODS
Subjects: This HIPPA compliant study [NCT04774471] was approved by the IRB. To-date 23 female volunteers (>18 years) who were scheduled for biopsy of a BIRADS 3, 4, or 5 breast lesion underwent non-contrast breast MRI after giving informed consent. All patients had undergone either a MG, an US, or both. One patient with high-risk had a lesion discovered on screening DCE-MRI.MRI Acquisition: Diffusion weighted images with fat saturation were acquired with two b-values (0 and 700 s/mm2) applied along 30 different directions, GRAPPA factor=2, an echo time = 90 ms, and corresponding diffusion time of 40 ms. Axial scans of both breasts were obtained with 2x2x2 mm3 resolution. Dynamic Field Correction (DFC) was applied which increased the acquisition time by ~3 minutes with a total time of ~8 min.
MRI Analysis: The diffusion weighted images were processed using BIT-Motion software (DDE MRI Solutions Inc., Tel Aviv, Israel) to evaluate multiple parameters including the three Eigenvalues λ1, λ2, λ3 and mean diffusivity (MD). The parametric maps were also co-registered with non-fat suppressed T2 weighted images. The maps and quantitative values of λ1, MD/ADC, as well as the fractional anisotropy (FA) and maximal anisotropy (λ1-λ3) were reviewed by two board certified breast radiologists each with greater than 10 years of experience in conjunction with the MG and/or US images. Regions of interest (ROIs) were manually defined in the lesions and in control region, i.e. unaffected breast parenchyma. The lesions were also characterized visually in terms of detectability using a four point scale, as well as showing “evidence of” or “absence of” malignancy on the DTI-derived maps. Scan interpretations were then compared with the final pathology.
Statistical methods: We evaluated differences between groups using Student’s T-test.
RESULTS
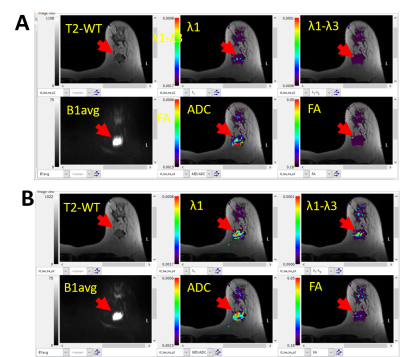
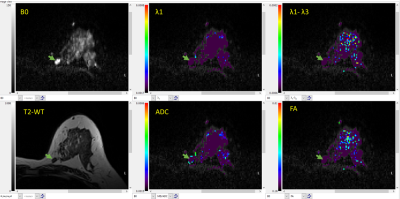
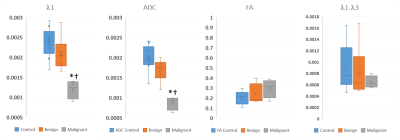
Visual Inspection: Two scans were non-interpretable: one malignant and one benign due to sub-optimal fat saturation. λ1 and ADC maps of the malignant lesions indicated evidence of malignancy (e.g. Figure 1.B). Many benign lesions were not uniquely visible, primarily because the diffusion parameters were similar to unaffected parenchyma as illustrated in Figure 2. Figure 1 also illustrates the need for DFC for DTI data acquisition, even though it results in acquisition time penalty.Quantiative analysis: ROI values of the DTI parameters in the malignant lesions were low (λ1=1.20±0.23; MD=0.87±0.53; FA=0.30±0.009; (λ1-λ3)=0.65±0.11), compared to a control area (λ1=2.36±0.38; MD=1.98±0.32; FA=0.20±0.004; (λ1-λ3)=0.91±0.39), and compared to the six other benign lesions (λ1=2.10±0.42; MD=1.69±0.27; FA=0.24±0.010; (λ1-λ3)=0.816±0.45) as demonstrated in Figure 3. Note units for λ1, MD and (λ1-λ3) are 10-3 mm2/s. FA and (λ1-λ3) showed no significant difference between the malignant lesions, benign lesions, or normal breast tissue (Figure 3).
DISCUSSION & CONCLUSION
This unique prospective study evaluated non-contrast MRI by DTI of indeterminate breast lesions against histopathology as the gold standard. DTI was performed applying 30 diffusion gradient directions, using 8 mm3 isotropic voxels and a diffusion time of 40 ms which substantially differ from those applied in a recent report [5] (6 diffusion gradient directions, 11.25 mm3 voxels, lower diffusion time, and performed post contrast). The longer diffusion time was chosen to optimize the DTI sensitivity to differentiate ductal/glandular anisotropy from malignant tissue vs TE [2]. Our experience supports the use of DFC during data acquisition [Figure 1]. While the acquisition time was substantially longer than the prior report (~8 vs. 3.5 min), motion did not compromise image quality.Our data suggest that the threshold values used in BIT-Motion software can reliably differentiate malignant from benign lesions by simple visual assessment of ADC and λ1 maps. Our quantitative analysis further validates the threshold values for displaying ADC and λ1 maps.
Most benign lesions show up iso-intense compared to fibrograndular tissue limiting their visibility. However, as shown in Figure 3, the distinct differentiation in λ1 and ADC values, allow for separating malignant lesions from benign tissues.
In conclusion, DTI parametric maps generated by the BIT-Motion software can characterize lesions previously identified on MG and/or US as malignant or benign eliminating the need for many unnecessary biopsies. Further study in a larger patient cohort is warranted and is currently in progress.
Acknowledgements
We thank Prof. Hadassa Degani for her helpful support and discussions. Work supported in part by the Washington Square Health Foundation.References
1. Clauser P, Krug B, Bickel H, Dietzel M, Pinker K, Neuhaus VF, Marino MA, Moschetta M, Troiano N, Helbich TH and Baltzer PAT. Diffusion-weighted Imaging Allows for Downgrading MR BI-RADS 4 Lesions in Contrast-enhanced MRI of the Breast to Avoid Unnecessary Biopsy. Clin Cancer Res. 2021;27:1941-1948.
2. Furman-Haran E, Grobgeld D, Nissan N, Shapiro-Feinberg M and Degani H. Can diffusion tensor anisotropy indices assist in breast cancer detection? J Magn Reson Imaging. 2016;44:1624-1632.
3. Shapiro-Feinberg M, Weisenberg N, Zehavi T, Furman-Haran E, Grobgeld D, Nissan N and Degani H. Clinical results of DTI. Eur J Radiol. 2012;81 Suppl 1:S151-2.
4. Amornsiripanitch N, Bickelhaupt S, Shim HJ, Dang M, Rahbar H, Pinker K and Partridge SC. Diffusion-weighted MRI for Unenhanced Breast Cancer Screening. Radiology. 2019;293:293-504.
5. Luo J, Hippe DS, Rahbar H, Parsian S, Rendi MH and Partridge SC. Diffusion tensor imaging for characterizing tumor microstructure and improving diagnostic performance on breast MRI: a prospective observational study. Breast Cancer Res. 2019;21:102.
Figures