1670
The relationship between cardiac fiber structure and regional function after myocardial infarction in rats1Institute for Experimental Medical Research, University of Oslo and Oslo University Hospital, Oslo, Norway, 2KG Jebsen Center for Cardiac Research, University of Oslo, Oslo, Norway, 3Oslo New University College, Oslo, Norway
Synopsis
Little is known about the relationship between cardiac function and myocardial fiber structure after myocardial infarction (MI). We hypothesized that post-MI remodeling of left ventricular fiber structure would be reflected in changes in the regional strains. Strains and cardiac fiber structure were measured using MRI in five rats with MI, and three sham-operated rats. Transmural variation in fiber helix angles was reduced adjacent to the infarct. This reduction was associated with reduced radial, longitudinal, and endocardial circumferential strain, and improved epicardial circumferential strain in the adjacent zone. Our findings emphasize the relevance of cardiac fiber structure remodeling after myocardial infarction.
Introduction
Regional myocardial function after myocardial infarction (MI) depends on the myocardium’s proximity to the infarct1-3. Recent studies on post-MI cardiac muscle fiber structure has revealed a reduction in the transmural variation in myocardial fiber helix angles in the viable left ventricular (LV) myocardium4. However, very little is known about the relationship between post-MI cardiac function and fiber structure.We hypothesized that a post-MI reorganization of the LV muscle fibers would be reflected in changes in the regional strains.
Methods
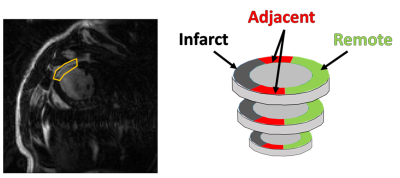
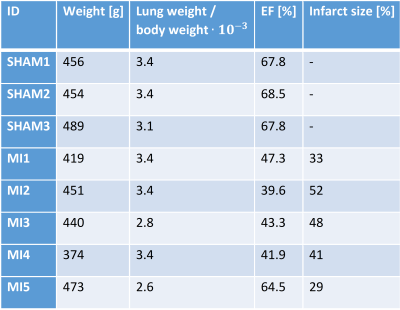
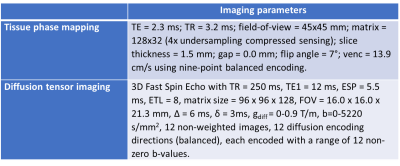
To test our hypothesis, MI was induced in five Wistar rats by left coronary artery occlusion. All infarctions were situated in the anterior LV free wall and were fully transmural, with MI sizes of 29-52 % (Table 1). Three sham-operated animals served as control. The animals used in this study were cared for according to the Norwegian Animal Welfare Act. The use of animals was approved by the Norwegian Animal Research Authority (FOTS ID 3284/10102). All in vivo MRI images were acquired on a 9.4T T/210 mm/ASR horizontal bore magnet (Agilent Technologies Inc., Santa Clara, CA, USA) with a volume transmit coil (inner diameter 72 mm) and a 4-channel phase array coil (Rapid Biomed GmbH, Rimpar, Germany). Details on sequences and scanning parameters can be found in Table 2. We used late gadolinium enhancement to measure infarct localization two days after operation. Global cardiac function was measured six weeks after operation using CINE. Regional LV cardiac strains were measured at the same time point using tissue phase mapping MRI. A stack of 11 slices covering the LV of the rat heart were acquired, with no gaps. The animals were anesthetized using isoflurane during MRI scans. Myocardial fiber structure was measured ex vivo in formalin fixated hearts using diffusion tensor imaging MRI. A volume transmit/receive coil with an inner diameter 19 mm (Rapid Biomed GmbH, Rimpar, Germany) was used for the ex vivo imaging. For the image analysis, we divided the non infarcted myocardium into two circumferential zones: the adjacent zone (1/6 of the myocardium on each side of the infarct), and the remote zone (4/6) (Figure 1). We segmented the images from the sham animals mimicking infarcts of similar size and location as in the MI animals. Mean fiber helix angles were calculated in five transmural layers in the remote and adjacent zone. The transmural helix angle gradient (THAG) was determined by fitting a line to the helix angles as a function of transmural depth percentage.Results
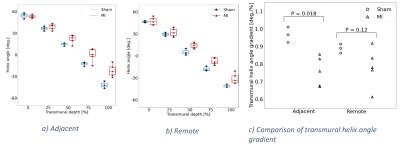
We found that the median left ventricular ejection fraction in the MI group (43.3 %) was lower than in the sham group (67.8 %) (Mann-Whitney U=0.0, P=0.018). THAG was reduced in the adjacent zone in the MI group compared to sham (Mann-Whitney U=0.0, P=0.018), whereas no differences were found in the remote zone (Figure 2). We found no dependence between THAGs and strains in the remote zone. However, in the adjacent zone, lower THAGs was associated with smaller longitudinal and radial strains (Pearson R=-0.71, P=0.05 and R=-0.75, P=0.03 respectively) (Figure 3a,b). Since circumferential strain vary transmurally, we investigated the correlations between THAGs and endocardial, midmyocardial and epicardial circumferential strain individually. In the adjacent zone, lower THAGs were associated with smaller circumferential strains in the endocardium (Pearson R=-0.74, P=0.04) (Figure 3c), and larger circumferential strains in the epicardium (Pearson R=0.85, P=0.008) (Figure 3e). No dependency was observed for mid myocardial circumferential strains (Figure 3d).Discussion
Here we show that the degree remodeling of fiber structure depends on proximity to the infarct in the post-MI rat (Figure 2). Reduced THAG was associated with smaller longitudinal strain in the adjacent zone. The shift towards more circumferential fibers in the epicardial half of the wall could contribute to this. Interestingly, we found the dependence between strain and fiber remodeling in the adjacent zone to vary between transmural layers. In the endocardium, reduced THAG was found to be associated with smaller circumferential strain. This is expected with the shift towards more longitudinal (and thus less circumferential) fibers in the endocardial half of the wall. In the epicardium, however, reduced THAG was associated with improved circumferential strain, which could reflect the shift towards more circumferential fibers in the epicardial half of the wall. Despite the shift towards more longitudinal fibers in the midmyocardium, no correlation was observed between THAG and circumferential strain in this layer.Finally, we found that reduced THAG was associated with smaller radial strain in the adjacent zone. Radial strain is a consequence of the limited compressibility of the myocardium as the ventricular wall shortens in the longitudinal and circumferential direction5. In the healthy heart, the lower circumferential strain in the epicardium compared to the endocardium contributes to radial stretch. The observed correlation between radial strain and THAG in the adjacent zone is therefore expected with a reduction in longitudinal strain and transmural homogenization in circumferential strain with reduced THAG.
Conclusion
Here we show that the degree of remodeling of cardiac fiber structure depends on the myocardium’s proximity to the infarct in the post-MI rat. The degree of remodeling was associated with increased transmural homogenization in circumferential strain. Our findings emphasize the relevance of cardiac fiber structure remodeling after myocardial infarction.Acknowledgements
This work was supported by KG JebsenCenter for Cardiac Research (Oslo,Norway), theSouth-Eastern Norway Regional Health Authority(Oslo, Norway), Familien Blix’ fond til fremme avmedisinsk forskning (Oslo,Norway), Olav Raagholtog Gerd Meidel Raagholts stiftelse for forskning(Oslo,Norway). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
1 Bogaert, J. et al. Remote myocardial dysfunction after acute anterior myocardial infarction: impact of left ventricular shape on regional function: A magnetic resonance myocardial tagging study. Journal of the American College of Cardiology 35, 1525-1534, doi:https://doi.org/10.1016/S0735-1097(00)00601-X (2000).
2 Götte, M. J. W. et al. Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. Journal of the American College of Cardiology 37, 808-817, doi:https://doi.org/10.1016/S0735-1097(00)01186-4 (2001).
3 Espe, E. K. S. et al. Regional Dysfunction After Myocardial Infarction in Rats. Circulation: Cardiovascular Imaging 10, e005997, doi:doi:10.1161/CIRCIMAGING.116.005997 (2017).
4 Mekkaoui, C. et al. Fiber architecture in remodeled myocardium revealed with a quantitative diffusion CMR tractography framework and histological validation. Journal of Cardiovascular Magnetic Resonance 14, 70, doi:10.1186/1532-429X-14-70 (2012).
5 Stokke, T. M. et al. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. Journal of the American College of Cardiology 70, 942-954, doi:https://doi.org/10.1016/j.jacc.2017.06.046 (2017).
Figures