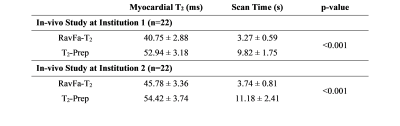1664
Rapid Variable Flip-angle T2 Quantification (RavFa-T2) of the Myocardium using Steady-state Free Precession: A Two-Center Study1Harvard Medical School, Boston, MA, United States, 2Boston Children’s Hospital, Boston, MA, United States, 3Technical University of Munich, Munich, Germany, 4Philips Healthcare, Boston, MA, United States, 5University of Colorado, Aurora, CO, United States, 6Children’s Hospital Colorado, Aurora, CO, United States
Synopsis
We developed a novel method (RavFa-T2) for T2 quantification of myocardium using transient steady-state free precession (SSFP) with a variable flip angle scheme. RavFa-T2 was systematically analyzed based on a phantom and a two-center patient study. We show that RavFa-T2 yields accurate T2 estimates for the myocardium compared to the state-of-the-art method T2-Prep and reduces scan time to ≤4 seconds.
Summary of Main Findings
RavFa-T2 combines a transient SSFP sequence and a variable flip angle scheme for myocardial T2 quantification at a rapid scan time (≤4 seconds). It showed accurate T2 quantification and lower T2 precision than T2-Prep in a phantom study. The precision of RavFa-T2 in-vivo was higher than T2-Prep.Introduction
Transverse relaxation times (T2) have long been used for tissue characterization of the myocardium in cardiovascular magnetic resonance imaging. Increased myocardial T2 values have been shown to correlate with myocarditis 1 and edema. 2 A conventional approach for assessing myocardial T2 is based on a method called T2-Prep with different delays of a single-shot steady-state free precession (SSFP) imaging sequence. 3 This technique, however, requires a long breath-hold acquisition time of 12 seconds, which can be demanding for very young or ill cardiac patients. To reduce breath-hold time, we developed a new method to quantify T2 of the myocardium in ≤4 seconds.Methods
The proposed RavFa-T2 pulse sequence combines transient SSFP imaging,4,5,6 and a variable flip angle scheme. 7,8 Compared to T2-Prep (Figure 1A), four images are acquired with variable flip angles ($$$\alpha_i= 30°, 67°, 103°, 140°$$$ ) at a specific cardiac phase in 4 heartbeats (Figure 1B). Assuming heartrates between 60-120 bpm, the acquisition time is 2-4 seconds. To account for partially recovered magnetization from previous images, we introduce an additional, iterative term $$$\delta_i$$$ to the transient phase SSFP relaxation model6:$$$M_{RavFa} (\alpha_i) = \delta_i \times M_{trans}(\alpha_i)$$$,
where $$$M_{trans}(\alpha_i)=\left( M_0sin(\frac{\alpha_i}{2})-M_{SS}(\alpha_i)\right) \lambda(\alpha_i)^n+M_{SS}(\alpha_i)$$$
with $$$ M_{SS}(\alpha_i) = M_0 \frac{\sqrt{E_2}(1-E_1)sin(\alpha_i)}{1-(E_1-E_2)cos(\alpha_i)-E_1E_2} $$$, $$$\lambda(\alpha_i)=E_1cos^2(\frac{\alpha_i}{2})+E_2sin^2(\frac{\alpha_i}{2})$$$, $$$E_{1/2}=exp(-\frac{T_R}{T_{1/2}})$$$,
and $$$\delta_1 = M_0$$$, $$$\delta_i=M_0-(M_0-M_{RavFa}(\alpha_{i-1})cot(\frac{\alpha_{i-1}}{2}))exp(-\frac{t}{T_1})$$$
where, t describes the time between heartbeats, TR is the repetition time, and n was fitted to match the underlying magnetization. To reduce the number of unknown parameters, we normalize the pixel intensities by dividing them by the corresponding pixel of the last image. This eliminates the term for proton density M0.
We systematically investigated the feasibility of the novel RavFa-T2 approach with a phantom and a two-center in-vivo study. The phantom study was performed to compare the proposed sequence to T2 of the gold-standard spin echo (SE) (TR/TE: 10000/32*10ms, α: 90°, total acquisition time (TAT): 4920 seconds), and T2-Prep (TR/TE: 2.65/1.33ms, α: 35°, rest period: 2 heartbeats, T2-Prep delay: 0, 25, 50, and 75ms, TAT: ≤12s). The experiments were performed on the T1MES phantom 9 at 4 different heartrates (60, 80, 100, 120 bpm) with a field-of-view (FOV): 200 (RL) × 200 (AP) × 10 (FH) mm, CS-SENSE 3, and 10 startup pulses for all methods. We performed a two-center in-vivo feasibility study in 44 patients using the same imaging parameters as in the phantom study. All scans were performed on 1.5T Philips scanners. The quantification algorithms were implemented in MATLAB (MathWorks Inc., Natick, MA, USA). Method evaluation was based on accuracy (mean error compared to SE) and precision (pixel-wise standard deviation within region-of-interest). A two-tailed paired student t-test was used for statistical analysis and a p-value≤0.05 was assumed statistically significant.
Results
Figure 2 shows the T2 maps of the phantom in all sequences. For the phantom study, the gold-standard SE yielded the following ranges for T2 = [48-235ms]. T2 quantification with T2-Prep demonstrated an accuracy/precision of 12.3ms/3.9ms compared to SE. The comparison of RavFa-T2 to SE resulted in an accuracy/precision of -9.4ms/12.5ms (Table 1). Forty-four patients at institution 1 (n= 22, 14 female, median age 28.5 [11-60] years old) and institution 2 (n = 22, 10 female, median age 19 [6-41] years old) successfully completed T2-prep and RavFa-T2 acquisitions in a mid-ventricular short-axis slice. Figure 3 demonstrates the T2 maps of 2 patients calculated with T2-Prep and RavFa-T2. For the two-center in-vivo study, the acquisition time of RavFa-T2 was consistently shorter than T2-Prep (Table 2, p-value <0.001) and the precision of RavFa-T2 was better than T2-Prep (Table 2, all p-values <0.001).Discussion
RavFa-T2 allows rapid myocardial T2 quantification in 4 heartbeats (≤4 seconds), and its acquisition time is at least 3 times faster than that of T2-Prep. In the phantom study, RavFa-T2 shows higher accuracy and lower precision compared to T2-Prep. However, the precision of RavFa-T2 is significantly better than T2-Prep in in-vivo study partially due to the shorter acquisition time of RavFa-T2. Compared to 12 second T2-Prep acquisitions, patients were able to completely hold their breath during the 4 second RavFa-T2 acquisitions, leading to a minimum drift in respiratory motion and better precision in T2 estimation.Conclusion
We demonstrate technical feasibility of myocardial T2 mapping with RavFa-T2. The estimation of myocardial T2 in the phantom results in accurate values with acceptable precision compared to T2-Prep, whereas in in-vivo study, results in better precession compared to T2-Prep. Future studies aim at extending RavFa-T2 method for simultaneously estimation of T1 and T2 of myocardium.Acknowledgements
This project was supported by the Harvard Scholarship Foundation Germany e.V.References
| 1. | Spieker M, Haberkorn S, Gastl M, et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. Journal of Cardiovascular Magnetic Resonance. December 2017;19. |
| 2. | Nishii T, Kono AK, Shigeru M, et al. Cardiovascular magnetic resonance T2 mapping can detect myocardial edema in idiopathic dilated cardiomyopathy. The International Journal of Cardiovascular Imaging. April 2014;30. |
| 3. | Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. Journal of Cardiovascular Magnetic Resonance. December 2009;11:56. |
| 4. | Oppelt A, Graumann R, Barfuss H, Fischer H, Hartl W, Schajor W. FISP: eine neue schnelle Pulssequenz fuer die Kernspintomographie. Electromedica. 1986;54(1):15-18. |
| 5. | Bieri O, Scheffler K. Fundamentals of balanced steady state free precession. Journal of Magnetic Resonance Imaging. 2013;38:2-11. |
| 6. | Scheffler K. On the Transient Phase of Balanced SSFP Sequences. Magnetic Resonance in Medicine. April 2003;49(4):781-783. |
| 7. | Worters P, Hargreaves B. Balanced SSFP transient imaging using variable flip angles for a predefined signal profile. Magnetic Resonance in Medicine. November 2010;64(5):1404-1412. |
| 8. | Srinivasan S, Ennis DB. Variable flip angle balanced steady-state free precession for lower SAR or higher contrast cardiac cine imaging. Magnetic Resonance in Medicine. March 2014;71(3):1035-1043. |
| 9. | Captur G, Captur G, Gatehouse PD, et al. A T1 and ECV phantom for global T1 mapping quality assurance: The T1 mapping and ECV standardisation in CMR (T1MES) program. Journal of Cardiovascular Magnetic Resonance. 2016;18(1):1-3. |
Figures
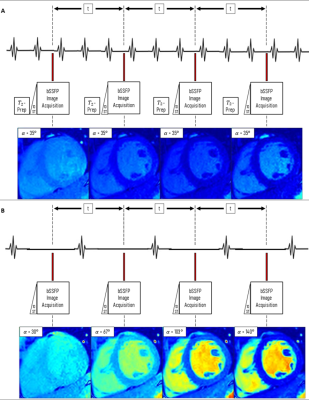
A) T2-Prep pulse sequence acquisition scheme (TR/TE: 2.65/1.33ms, α: 35°, rest period: 2 heartbeats, T2-Prep delay: 0, 25, 50, and 75ms). Image acquisition is performed during breath-hold in ≤12s; and B) RavFa-T2 pulse sequence acquisition scheme. Four images are acquired in the same diastolic phases within 4 consecutive heartbeats (t). Each image is acquired with a different flip angle (α = [ 30°, 67°, 103°, 140°]) after ten linear startup pulses (ST). All further sequence parameters are kept constant (TR/TE: 2.96/1.48ms). Image acquisition is performed during breath-hold in ≤4s.

T2 maps of T1MES phantom. T2 maps for SE, T2-Prep, and RavFa-T2. T2 values in ms of SE are displayed right of the respective tubes.


Results of the phantom study. (Top) T2 values for the 9 tubes of the T1MES phantom at 4 different heartrates (60 bpm, 80 bpm, 90 bpm, 120 bpm) as mean ± standard deviation over pixel values in region-of interest. (Bottom) The accuracy (mean error) and precision (pixel-by-pixel standard deviation) of T2-Prep, and RavFa-T2 compared to gold-standard SE.