1660
Effects of warm ischemia on myocardial metabolism: a normothermic perfused heart MRS study1Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 2Oxford Centre for Magnetic Resonance, University of Oxford, Oxford, United Kingdom, 3MRC Mitochondrial Unit, University of Cambridge, Cambridge, United Kingdom, 4Department of Surgery, University of Cambridge, Cambridge, United Kingdom, 5Royal Papworth Hospital, Cambridge, United Kingdom, 6Royal Veterinary College, London, United Kingdom, 7Department of Radiology, University of Cambridge, Cambridge, United Kingdom
Synopsis
Ischaemia-reperfusion injury is a key pathophysiological process driving cardiac injury in myocardial infarction, heart surgery and transplants. Succinate accumulation is a specific marker of ischaemic injury, and a potential therapeutic target to reduce reperfusion injury.Single-voxel proton spectra were acquired during normal perfusion and during ischaemia in three machine-perfused porcine hearts. During ischaemia, a peak was evident in the expected region of succinate, which was absent during normal perfusion. This was further examined in a phantom model which confirmed the peak location with concentration-signal correlation of R>0.999. MRS detection of succinate holds potential for the detection of ischaemia.
Introduction
A shortage of suitable donor organs limits the number of heart transplants that can be performed. Using normothermic machine perfusion (NMP), donated hearts can be preserved in a near-physiological environment while they are ex-situ. This enables the transplantation of hearts from circulatory death donors, previously considered too high-risk. However, a set of sensitive and specific biomarkers is needed to carefully assess each organ’s viability for transplantation.Recent work has demonstrated succinate to be a key metabolic marker of ischaemia showing intracellular build up during ischaemia and rapid oxidation and efflux following re-oxygenation. Thus, its detection/quantification holds great clinical implications.
In vivo detection of succinate by magnetic resonance spectroscopy (MRS) has been reported in the brain and in some tumours1–3. However, no studies have reported it within a metabolically-active heart. Therefore, we combined an NMP device with a 3T MRI to examine the feasibility of detecting cardiac succinate by MRS in ex-vivo porcine hearts.
Methods
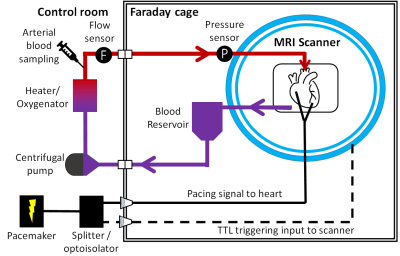
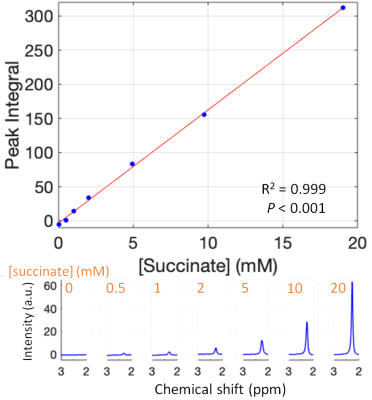
Phantom experiment: A phantom was prepared in a 250-mL pear-shaped flask with succinic acid (Sigma Aldrich) and distilled water. 1H-MRS was then acquired using PRESS-WSC4 from a 20x20x20mm3 voxel in the centre of the phantom for seven concentrations (0-20 mM). The data were analysed and fitted offline5.Perfusion experiment: A clinical cardiac NMP device (mOrgan, Royal Papworth Hospital, UK) was made MR-compatible by extending the perfusion lines and moving all metallic components to outside the scanner’s Faraday cage (Figure 1). Hearts from three white Landrace pigs were retrieved following the standard protocol for a human donor following circulatory death. Following extraction, the aorta was cannulated and antegrade perfusion (Langendorff model) was started. Perfusion was briefly paused while the perfusion lines were passed through the MRI scanner waveguide.
The hearts were paced 10 bpm faster than its normal rate using a clinical epicardial pacing device (Reocor D, Biotronics) with wires fed through a low pass filter. An optoisolated TTL version of the pacing signal was fed into the scanner’s external trigger input to enable cardiac gating.
The hearts were scanned on a 3T scanner (Prisma, Siemens Healthineers, Germany) with a 32Rx head coil. MR scanning commenced 4-5 hours after retrieval.
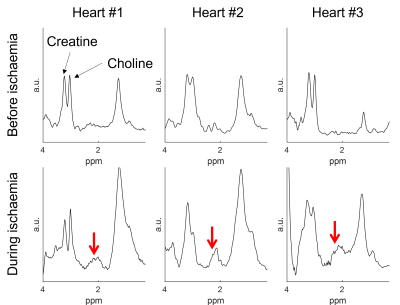
1H-MRS were collected from the septum with PRESS-WSC as previously described4, except TE=144 ms was used to reduce lipid contamination. Sixty-four averages were acquired for each water-suppressed scan. Each heart underwent baseline scans before a period of warm ischaemia (15-60 min) was induced by halting the perfusion. A repeat set of spectra was collected during warm ischaemia. All spectra were post-processed using OXSA5. Biopsies were also obtained at the end of each ischaemic period.
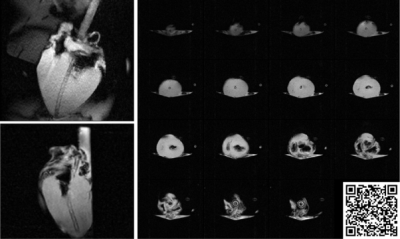
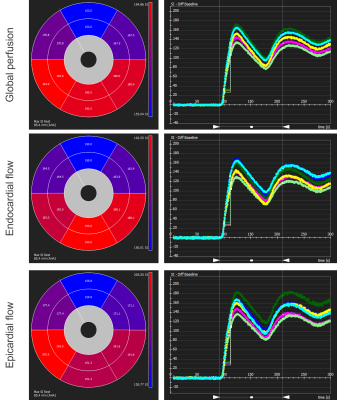
A first-pass perfusion image was obtained using turbo-FLASH with the following parameters: TE=1.2ms; TR=157.9ms; 3 slices(basal, mid, apical ventricle in short axis). 5 mL of Gadovist was injected into the blood reservoir. Images were analysed in CVI42 (Circle Cardiovascular Imaging, Calgary). As this irreversibly alters tissue contrast and must be performed with the heart in sinus rhythm, it was only completed in heart #1.
Results
The correlation between succinate’s peak integral and the actual concentration in the phantom is shown in Figure 3. Succinate’s chemical shift in the phantom is 2.3 ppm.MRS data before and during ischaemia is shown in Figure 4. In the ischaemic spectra, a broad peak between 2.1 and 2.4 ppm was seen in all hearts. Perfusion data is shown in Figure 5 and there was no epicardial-endocardial gradient.
Discussion
This study introduces a novel setup to study ex-situ donor hearts during NMP. This allows a comprehensive MRI examination with concurrent serum sampling and biopsies.The PRESS-WSC sequence accurately quantified succinate in the phantom and detected succinate in the myocardium during warm ischaemia. The peak between 2.1-2.4 ppm is rather broad, and we believe that other metabolites, e.g. acetyl-carnitine (2.1 ppm), might be present.
These results will require a larger study to determine thresholds in which ischaemia become detectable with PRESS-WSC. However, if confirmed, these results hold significant clinical implications. Succinate is a specific marker of ischaemia and plays a direct role in reperfusion injury6. Animal models have shown this to be a modifiable marker, with pre-treatment with malonate reducing succinate accumulation and infarct size6,7. Succinate accumulation is also associated with transplant organ damage8. Thus, a non-invasive technique to study this metabolite and its therapy response will be of great utility.
Although measuring first-pass perfusion is feasible in the NMP setup, the persistence of contrast following administrations impacts all following sequences and hence non-contract alternatives would be preferable.
Replication of the current studies findings will be sought in ex-situ human hearts, for which we have ethical approval. Further protocol optimisation,e.g. adding relaxometry, will provide a holistic assessment of the NMP heart for future experiments.
Conclusion
This study introduces a novel MR-compatible clinical perfusion system for non-invasively studying cardiac metabolism and pathology ex-situ. 1H-MRS detection of succinate holds potential for the detection of ischaemia. This will aid the optimisation of future perfusion devices and increase our understanding of cardiac injury and recovery following ischaemia.Acknowledgements
In this work, BD and LY are joint first authors; CTR and JRWM are joint senior authors.
BD is funded by Gates Cambridge. MH is funded by Wellcome Trust (220581/Z/20/Z). CTR is funded by the Wellcome Trust and Royal Society (098436/Z/12/B). JRWM and this research was supported by the Cambridge BHF Centre of Research Excellence [RE/18/1/34212]. This research was also supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- Helman, G. et al. Magnetic resonance imaging spectrum of succinate dehydrogenase-related infantile leukoencephalopathy. Annals of Neurology 79, 379–386 (2016).
- Lussey-Lepoutre, C. et al. In Vivo Detection of Succinate by Magnetic Resonance Spectroscopy as a Hallmark of SDHx Mutations in Paraganglioma. Clin Cancer Res 22, 1120–1129 (2016).
- Garg, M. et al. Brain abscesses: etiologic categorization with in vivo proton MR spectroscopy. Radiology 230, 519–527 (2004).
- Ding, B. et al. Water-suppression cycling 3-T cardiac 1 H-MRS detects altered creatine and choline in patients with aortic or mitral stenosis. NMR Biomed 34, e4513 (2021).
- Purvis, L. A. B. et al. OXSA: An open-source magnetic resonance spectroscopy analysis toolbox in MATLAB. PLoS One 12, e0185356 (2017).
- Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
- Valls-Lacalle, L. et al. Selective Inhibition of Succinate Dehydrogenase in Reperfused Myocardium with Intracoronary Malonate Reduces Infarct Size. Sci Rep 8, 2442 (2018).
- Martin, J. L. et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat Metab 1, 966–974 (2019).
Figures