1657
Sample Extraction of ex vivo Myocardial Tissue: Combination of High Resolution MRI with 3D-printed Guides1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Dept. of Cardiology and Angiology I, University Hospital Freiburg and Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
Magnetic resonance provides a multitude of imaging techniques to detect and characterize myocardial infarction (MI). Terminal animal studies are often performed in which CMR findings after MI are correlated to histology. However, precise manual extraction of tissue samples from a specific myocardial region defined by CMR is difficult. Here, we propose the use of 3D-printed guides for extraction of tissue samples from myocardial regions pre-defined on high resolution ex vivo CMR. Using the presented technique, histology findings can be correlated to the exact position within the MRI data set with a precision of < 1 mm.
Introduction
Cardiovascular magnetic resonance (CMR) provides a multitude of imaging techniques to detect and characterize myocardial infarction (MI). In particular, relaxometry and feature tracking based on diffusion-weighted imaging are increasingly used to gain insight into the morphology and dynamics of MI1,2. Therefore, terminal animal studies are often performed in which CMR findings after experimentally induced MI are correlated to histology3–5. However, manual extraction of tissue samples from a specific myocardial region defined by CMR is difficult due to the lacking spatial correlation between the extracted tissue and MRI. Here, we propose the use of individualized 3D-printed guides to enable precise extraction of tissue samples from myocardial regions of interest that are pre-defined on CMR.Methods
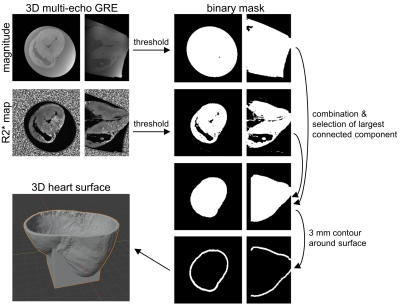
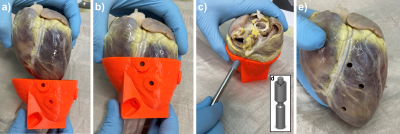
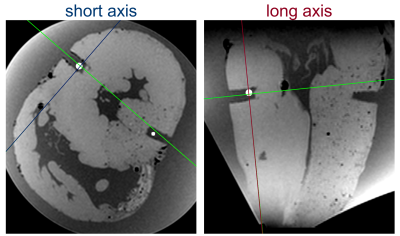
In this study, four pig hearts were excised after the animals underwent an interventional CMR study6,7. During this study, a contrast agent based on magnetic particles of iron oxide (MPIO)8–10 was injected in the left coronary artery of the pigs. After termination, the hearts were excised within 60 minutes, flushed and stored in a cylindrical plastic container (diameter: 10 cm, height: 12 cm) filled with formaldehyde. To enable diffusion of the fixation through the myocardium the hearts were left in the solution for at least 7 days before high resolution ex vivo MRI was performed. High resolution MRI of the excised hearts was performed at 3T system (Siemens PrismaFit) using a 64 channel head/neck coil. A multi-echo spoiled GRE data was acquired of the whole heart (TR = 23 ms, TE = [3.4,9.8,17.1] ms, FA = 12°, BW = 260 Hz/px, FoV = 129x129x84 mm³, matrix = 224x224x144, averages: 2). The magnitude images and the R2* maps calculated from the three echoes were used for both visualizing the MPIO contrast agent distribution and creating an individual digital 3D heart model. The post-processing to obtain the models was done in Matlab and the pipeline is illustrated in Fig. 1. In brief, magnitude and R2* thresholding are used to create a binary mask of the myocardium. Then, the largest connected component of the 3D mask is found and selected using the Matlab function bwconncomp to remove residual non-zero entries outside the myocardium. In addition, the ventricles and atria are thus added to the binary mask such that the myocardial mask has a single boundary surface. A 3 mm thick shell is then created around this surface which is used to hold the heart when tissue samples are extracted. The extraction is done using a custom-made cylindrical biopsy punch with 5 mm diameter and a maximum depth of 20 mm. The target locations are selected manually from the three-dimensional R2* maps. Here, multiple targets were selected for each heart from both remote areas were no MPIOs are seen and areas with high MPIO density (Fig. 2). Trajectories are calculated normal to the epicardial surface, and a 5 mm wide and 5 mm long cylindrical guide is created around each trajectory. The final models are then created by subtracting the guides from the shell and the addition of a stand. The models were printed in PLA using a Prusa I3 MK3S Printer. The post-processing is automated such that the only user input is the definition of the target points. To extract the samples, the punch is inserted to the known target depth plus 2 mm, and for confirmation the hearts are imaged again and co-registered to the baseline images. The precision of the sample extraction is then calculated as the lateral offset of the center of the sample relative to the target point in the baseline images (Fig. 4).Results
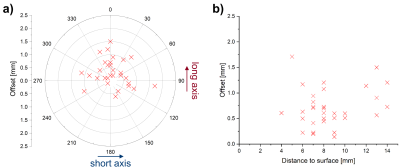
Average printing time was 7 hours per heart. Each 3D-printed guide fits the corresponding heart with no substantial clearance (Fig. 3). In total, 34 samples were extracted and all offsets were < 2.5 mm with the average being (0.70 ± 0.38) mm. Figure 5a shows a polar plot the offsets with respect to the short and long axes of the heart. The mean of the offset along the short axis was (0.08 ± 0.56) mm and not significantly different from zero (p = 0.4), whereas the mean offset along the long axis was significantly larger than zero (p = 0.001) with a mean of (0.29 ± 0.49) mm. Furthermore, no significant linear dependence of the offset on the distance of the target point to the surface was found (p = 0.35, Fig. 5b).Discussion & Conclusion
The presented method allows for the extraction of tissue samples from myocardial regions defined on high resolution ex vivo MRI with a precision that is below 1 mm on average. Using the presented technique, histology findings can be correlated to the exact position within the MRI data set. This can be particularly helpful for studies of myocardial infarction, where the tissue and thus relaxation and diffusion parameters measured with MRI appear heterogeneous. Furthermore, the proposed method can be extended to ex vivo studies of other organs and combined with techniques such as needle biopsy.Acknowledgements
This study is part of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation #422681845).References
1. Fernández-Jiménez, R. et al. Dynamic Edematous Response of the Human Heart to Myocardial Infarction. Circulation 136, 1288–1300 (2017).
2. Stoeck, C. T. et al. Cardiovascular magnetic resonance imaging of functional and microstructural changes of the heart in a longitudinal pig model of acute to chronic myocardial infarction. J. Cardiovasc. Magn. Reson. 23, 103 (2021).
3. Fernández-Jiménez, R. et al. Effect of Ischemia Duration and Protective Interventions on the Temporal Dynamics of Tissue Composition After Myocardial Infarction. Circ. Res. 121, 439–450 (2017).
4. Fernández-Jiménez, R. et al. Myocardial Edema After Ischemia/Reperfusion Is Not Stable and Follows a Bimodal Pattern: Imaging and Histological Tissue Characterization. J. Am. Coll. Cardiol. 65, 315–323 (2015).
5. Wang, Y., Cai, W., Wang, L. & Xia, R. Evaluate the early changes of myocardial fibers in rhesus monkey during sub-acute stage of myocardial infarction using diffusion tensor magnetic resonance imaging. Magn. Reson. Imaging 34, 391–396 (2016).
6. Heidt, T. et al. Real-time magnetic resonance imaging – guided coronary intervention in a porcine model. Sci. Rep. 9, 8663 (2019).
7. Heidt, T. et al. Magnetic resonance imaging for pathobiological assessment and interventional treatment of the coronary arteries. Eur. Heart J. Suppl. 22, C46–C56 (2020).
8. Heidt, T. et al. Molecular Imaging of Activated Platelets Allows the Detection of Pulmonary Embolism with Magnetic Resonance Imaging. Sci. Rep. 6, 25044 (2016).
9. Duerschmied, D. et al. Molecular Magnetic Resonance Imaging Allows the Detection of Activated Platelets in a New Mouse Model of Coronary Artery Thrombosis. Invest. Radiol. 46, 618–623 (2011).
10. von zur Muhlen, C. et al. Magnetic Resonance Imaging Contrast Agent Targeted Toward Activated Platelets Allows In Vivo Detection of Thrombosis and Monitoring of Thrombolysis. Circulation 118, 258–267 (2008).
Figures