1655
Rapid and Robust Assessment of Circumferential Strain Recovery via Tagged MRI of Infarcted Pig Hearts After Human Cardiomyocyte Transplantation1Radiology, University of Washington, Seattle, WA, United States
Synopsis
A new straight forward approach for rapid and robust quantification of myocardial circumferential strain has been developed. The key to this method is placement of the linear tags in 60-degree pattern offsets and alignment of the tags with the AHA segments for optimized segmental analysis. The approach has been implemented for the first time for evaluation of the human cardiomyocyte transplantation benefits in the infarcted pig heart. We have shown the temporal changes in circumferential strain and strain rate of the infarcted segments of myocardium and recovery of myocardial strain followed human cardiomyocyte transplantation in comparison with untreated control group.
Introduction
Recent studies have shown that intramyocardial delivery of human embryonic stem cell derived cardiomyocytes (hESC-CM) improved global left ventricle (LV) function and decreased remodeling after myocardial infarction (MI) of the hearts of non-human primates 1,2. Regional LV function of the infarcted heart after hESC-CM has not been explored. MR tagging technique is the non-invasive method for assessment of regional myocardial deformation. However, orthogonal orientation of tagging lines lack sensitivity and produce artifacts when the tagging pattern is tangential to the heart wall. Aim of the study was to develop a new rapid method for assessment of the regional myocardial deformation from MRI tagging images and to test the robustness of this technique for evaluation of the cell transplantation benefits in the large animal model of myocardial infarction.Methods
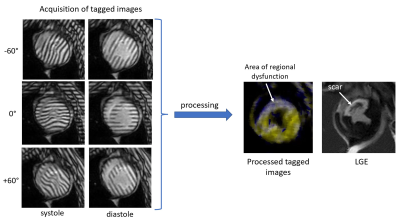
Animals. 13 castrated young male Yucatan minipigs weighing between 30-40 kg were included unto the study. Myocardial infarction (MI) was modeled using percutaneous ischemia/reperfusion 3.CMRI acquisition. In vivo cardiac MRI studies were conducted on a 3T Ingenia CX clinical scanner (Philips, Best, Netherlands) at the different time points: on healthy animals before MI modeling and then at 2, 4, 8, 12, and 16 weeks after MI. During the scan, the animals were sedated with a combination of Butorphanol, Acepromazine and Ketamine administered intramuscularly. Animals were then intubated and mechanically ventilated using Isoflurane and oxygen to maintain a surgical plane of anesthesia during the scan. CMRI protocol included a balanced turbo field echo (bTFE) cine sequence for assessment of the heart contractility; complimentary spatial modulation of magnetization (SCPAMM) sequence was used for tagging imaging; late gadolinium enhancement scan with phase-sensitive inversion recovery (PSIR) pulse sequence was used for measuring of the infarct size. Tagging lines were placed in the short axis of the heart tangential to the heart wall with 60-degree shift to each other and were aligned with the standard AHA segments (Figure 1). The parameters of the SCPAMM sequence were the following: TR 5.8ms; TE 3.5 ms; flip angle 10 degree; field of view 350x350 mm; slice thickness 8 mm; image resolution 1.1x1.1 mm; 3 mm tag separation. All acquisitions were done with 1 signal average and breath hold.
Tagging analysis. A new image processing technique for rapid analysis of tagged CMR images uses isolated spectral peaks in SCPAMM-tagged images, which contain information about cardiac motion. Frequency is estimated by local Fourier transformation (Figure 2). Custom-written MATLAB software analyzes circumferential shortening in the zones of circumferential sensitivity as shown in the figure 2. Measurements were combined geometrically for areas of overlap between patterns. Global as well as regional strain and strain rate in six AHA segments of one short-axis slice were measured.
Statistical Analysis. Statistical analyses were carried out in Excel software for Windows (Microsoft Inc., Redmond, WA, USA).
Results
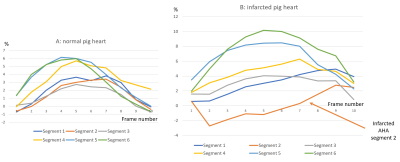
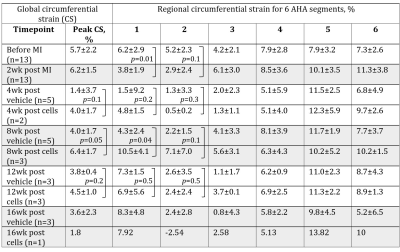
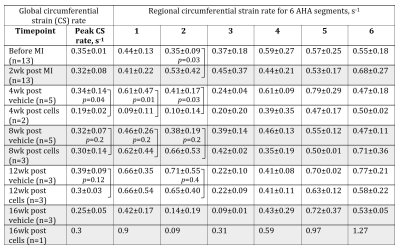
There was a decrease in circumferential strain (CS) and increase in CS rate in infarcted segments of the pig heart (Tables 1, 2). Specifically, CS decreased from 5.2±2.3% to 2.9±2.4% at two weeks after MI (p=0.01) in the infarct zone localization (AHA segment 2). The compensatory increase in CS was noticed in the non-infarcted segments 5 and 6 (Figure 3). CS rate increased at the same segment from 0.35±0.09 s-1 to 0.53±0.42 s-1 (p=0.03). Cell therapy causes increase of the global and regional CS in all studied time points and decrease in CS rate in the cell-treated group in comparison with untreated control.Discussion
Three orientations of tags at 60-degrees pattern aligned with AHA heart segments provide robust assessment of circumferential strain and strain rate. This new approach is fast and much less noisy than other methods of tagging analysis, such as HARP that directly use k-space to estimate “instantaneous” frequency 4. The novel approach was sensitive in detection of circumferential strain decrease after MI and its recovery after cell therapy. Statistical significance was not reached in some of the studied time points because of the small sample size.Conclusion
This study demonstrates a new simple approach for rapid and robust quantification of myocardial circumferential strain. The key to this method is placement of the linear tags in 60-degree pattern offsets and alignment of the tags with the AHA segments for optimized segmental analysis. This method has been implemented for the first time for evaluation of the human cardiomyocyte transplantation benefits in the infarcted pig heart. We have shown the temporal changes in circumferential strain and strain rate of the infarcted segments of myocardium and recovery of myocardial strain followed human cardiomyocyte transplantation in comparison with untreated control group.Acknowledgements
We appreciate Lauren E. Neidig, Emily Spaulding and Gary Fye for pig surgery, help with anesthesia and breath hold technique during CMRI studies. Dr. Kenta Nakamura for cell transplantation to the pig heart. Dr. Charles E. Murry for general support and funding.References
1. Chong JJ, Wang X, Don CW, et al. Human embryonic-stem-cell derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277.
2. Liu YW, Chen B, Yang X. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol.2018,36:597–605.
3. McCall FC, Telukuntla KS, Karantalis V, et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc. 2012;7(8):1479–1496.
4. Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048-1060.
Figures