1652
Single Breath-hold Simultaneous T2 and TRAFF2 mapping for approximate Spin-lock Dispersion Mapping in the Myocardium at 3T
Joao Tourais1, Omer Burak Demirel2, Qian Tao3, Iain Pierce4, George Thornton4, Thomas Treibel4, Sebastian Weingärtner1, and Mehmet Akcakaya2
1Imaging Physics, Delft University of Technology (TU Delft), Delft, Netherlands, 2Department for Electrical and Computer Engineering, and Center for Magnetic Resonance Research, University of Minnesota, Minnesota, MN, United States, 3Delft University of Technology (TU Delft), Delft, Netherlands, 4Barts heart centre, Barts Health NHS Trust, London, United Kingdom
1Imaging Physics, Delft University of Technology (TU Delft), Delft, Netherlands, 2Department for Electrical and Computer Engineering, and Center for Magnetic Resonance Research, University of Minnesota, Minnesota, MN, United States, 3Delft University of Technology (TU Delft), Delft, Netherlands, 4Barts heart centre, Barts Health NHS Trust, London, United Kingdom
Synopsis
Spin-lock dispersion is a promising biomarker for the assessment of myocardial infarction. However, at 3T, the required range of T1⍴ maps acquired at different amplitudes suffers from specific absorption rate (SAR) limitations and off-resonance artifacts. Relaxation Along a Fictitious Field (RAFF) is an alternative to SL preparations with lower SAR requirements while still sampling relaxation in the rotating frame. Thus, a single breath-hold simultaneous TRAFF2 and T2 mapping sequence is proposed for sl dispersion mapping at 3T. High visual quality maps and accurate T2, TRAFF2, and SL dispersion values are achieved with the proposed sequence in phantoms and in vivo.
Introduction
Spin-lock (SL) dispersion has recently been proposed as a new imaging biomarker for the detection of myocardial infarction1. SL dispersion is usually obtained by acquiring a range of T1⍴ maps at different amplitudes (frequencies). However, at 3T, specific absorption rate (SAR) limits the use of high-frequency SL pulses, and long SL preparations suffer from strong off-resonance induced artifacts. Adiabatic T1rho may be used to avoid off-resonance effects, but its use in dispersion mapping is hampered as variable effective field strengths and sweep durations complicate the relation to a well-defined SL frequency. Relaxation Along a Fictitious Field (RAFF)2 is an alternative to SL pulses with lower SAR requirements. RAFF operates in a sub-adiabatic regime with constant effective field strength and an identical, constant fictitious field strength leading to uniform sweeps. In previous studies, RAFF has been shown to preserve excellent sensitivity to acute myocardial infarction and chronic scar3, but its use for dispersion mapping remains unstudied. In this work, we sought to provide a method for the assessment of myocardial SL dispersion at 3T using RAFF in a single breath-hold. The proposed technique is evaluated in phantom measurements and initial in vivo images of a healthy subject.Methods
In the proposed simultaneous TRAFF2 and T2 mapping sequence, five balanced SSFP images with different magnetization preparations were acquired during a single 16s breath-hold (Fig. 1). A first image acquired with no preparation is followed by images prepared with two RAFF2 preparation blocks3 (TRAFF2P) and one T2 preparation (T2P) duration, each preceded by a 4s rest period. Additionally, a saturation-prepared image was acquired in the subsequent heartbeat to capture the effect of imaging pulses on the magnetization curve. Simultaneous TRAFF2 and T2 maps were obtained using a 3 parameter fit as follows:S = A * exp(-T / TRAFF) * exp(-T / T2) + B
SL dispersion maps were calculated by the difference between T2 and TRAFF2 times normalized by the RAFF frequency as the assumed SL frequency.Phantom and in vivo measurements were performed on a 3T MRI scanner (Prisma, Siemens Healthineers, Erlangen, Germany) with a 28-channel receiver coil array.Phantom measurements were performed on the T1MES4 phantom to evaluate the accuracy and precision of the proposed sequence against regular TRAFF2 and T2 mapping. Simultaneous TRAFF2 and T2 maps were obtained using the proposed sequence with TRAFF2P times of 12.9 and 25.7 ms (RF pulse power of 625 Hz) and T2P of 50 ms. To assess inter-scan reproducibility, 3 scan repetitions were performed in separate acquisitions in the phantom experiments and the coefficient of variation (CV) across all the measurements was computed. A reference TRAFF2 map was obtained using TRAFF2P = 0, 12.9, 25.7, and 38.6 ms and an image preceded by a saturation pulse. Reference T2 map was acquired with T2P of 0, 50, 50, 50 ms, followed by an image preceded by a saturation pulse5. Correlation and Bland-Altman plots were obtained to compare the joint and the individual TRAFF2, T2, and SL dispersion values.TRAFF2 and T2 maps were obtained, using the proposed RAFTS sequence and individual reference methods, for one healthy subject (male, 35y) in a breath-hold of 16s for each sequence. All baseline in vivo images were registered using group-wise registration to minimize motion within and across the separate breath-holds6 and SL dispersion was computed. Standard deviation (SD) maps in ms were obtained from the fit residuals5 to obtain a spatially resolved estimation of the precision of the proposed sequence.The imaging parameters were kept constant for phantom and in vivo measurements: FOV: 320x255mm2; In-plane resol.: 1.7x1.7 mm2; Slice thick.: 8mm; GRAPPA factor/Ref. lines: 2/24; TE 1.6ms; Readout type: bSSFP.
Results
Simultaneous TRAFF2 and T2 values obtained with the proposed sequence exhibited excellent correlation (R2 = 1.00) with the reference TRAFF and T2 for the phantom vials.Dispersion maps calculated based on TRAFF2 as an approximation for SL relaxation, and T2 maps as zero-frequency SL, yield good sensitivity to changes in the vials of the T1MES phantom (Fig. 2). In addition, excellent reproducibility (CV < 10% for the majority of the vials) was observed in the phantom measurements (Fig. 3) .In vivo TRAFF2 and T2 maps with the proposed sequence were obtained with high visual map quality, and no B1+ or B0 related artifacts were visible (Fig. 4-5). The average (± std) myocardial TRAFF2 and T2 obtained with the proposed sequence (68.0 ± 10.7 and 44.0 ± 4.0 ms, respectively) were comparable to the reference methods (62.7 ± 11.7 and 41.2 ± 2.4 ms, respectively). The myocardial SL dispersion map resulted in an excellent depiction of the myocardium with a small increase in variability when compared to the SL dispersion obtained with the single-parameter reference maps (0.4 ± 0.2 and 0.3 ± 0.2 x 10-4 s2, respectively).Conclusion
Our results indicate promising potential for myocardial spin-lock dispersion in a single breath-hold using TRAFF2 mapping with clinically tolerable SAR and without the use of contrast agents. The proposed sequence obtains simultaneous TRAFF2 and T2 maps and yields high visual image quality, homogeneous signal, no B1+ or B0 artifacts, and excellent reproducibility. Clinical sensitivity to pathological remodeling, such as scar, will be the subject of future studies.Acknowledgements
S.W. acknowledges funding from the 4TU Precision Medicine program, an NWO Start-up STU.019.024, and ZonMW OffRoad 04510011910073. M.A. acknowledges funding from the NIH R01HL153146, NIH P41EB027061, NIH R21EB028369References
[1] Han, Y., Liimatainen, T., Gorman, R.C. et al. (2014), Assessing Myocardial Disease Using T1ρ MRI. Curr Cardiovasc Imaging Rep 7, 9248.[2] Liimatainen, T., Sorce, D.J., O'Connell, R. et al. (2010), MRI contrast from relaxation along a fictitious field (RAFF). Magn. Reson. Med., 64: 983-994.[3] Yla-Herttuala, E., Laidinen, S., Laakso, H. et al. (2018), Quantification of myocardial infarct area based on TRAFFn relaxation time maps - comparison with cardiovascular magnetic resonance late gadolinium enhancement, T1ρ and T2 in vivo. J Cardiovasc Magn Reson 20, 34.[4] Tao, Q., van der Tol, P., Berendsen, F.F. et al. (2018), Robust motion correction for myocardial T1 and extracellular volume mapping by principle component analysis-based groupwise image registration. J. Magn. Reson. Imaging, 47: 1397-1405.[5] Jokivarsi KT, Liimatainen T, Kauppinen RA, et al. (2013), Relaxation Along a Fictitious Field (RAFF) and Z-spectroscopy using Alternating-Phase Irradiation (ZAPI) in Permanent Focal Cerebral Ischemia in Rat. PLOS ONE 8(7): e69157.Figures
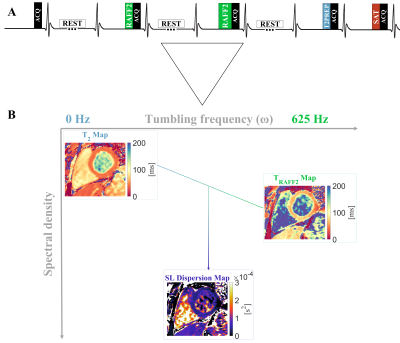
A) Pulse sequence diagram for the proposed simultaneous myocardial TRAFF2 and T2 mapping sequence. B) The difference between TRAFF2 (max frequency) and T2 (0 frequency) map divided by the RAFF2 pulse amplitude results in an approximation for a spin-lock (SL) dispersion map.
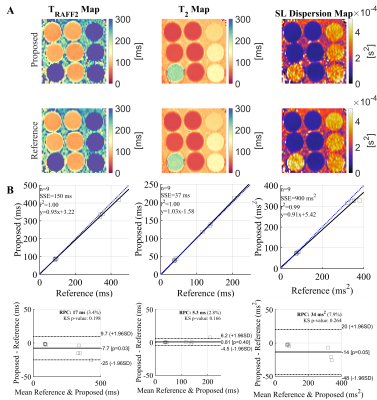
A) T2, TRAFF2, and spin-lock (SL) Dispersion maps (TRAFF2 - T2) for the T1MES phantom using the proposed simultaneous sequence and the single-parameter reference maps. B) Correlation and Bland-Altman plots for each parameter, showing an excellent correlation between the relaxation properties in phantom, in the expected in vivo range.

Coefficient of variation (CV) across 3 measurements using the proposed simultaneous TRAFF2 and T2 mapping sequence. All vials show a CV lower than 10% indicating excellent reproducibility of the proposed sequence.

A) Baseline images for the proposed simultaneous TRAFF2 and T2 mapping sequence with the corresponding preparation times. B) In vivo myocardial TRAFF2, T2, and spin-lock (SL) dispersion maps were obtained with the proposed sequence and with the single-parameter sequences (Reference). After image registration, the dispersion map is obtained by subtracting T2 from TRAFF2 and dividing by the RAFF2 pulse power. All maps present high visual image quality, homogeneous myocardial signal and no B1+ or B0 artifacts are visible.

Standard deviation (SD) maps obtained from the fit residuals, which indicate a spatially resolved estimation of the precision of the measured parameter.
DOI: https://doi.org/10.58530/2022/1652