1651
Isotropic fixed time 3D late gadolinium enhancement MRI for left atrial fibrosis imaging1Radiology & Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Biomedical Engineering, University of Utah, Salt Lake City, UT, United States, 3Internal Medicine, University of Utah, Salt Lake City, UT, United States, 4Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Left atrial late gadolinium enhancement imaging is a promising tool to identify scar and fibrosis. Existing acquisition and reconstruction methods can suffer from long scan time, poor image quality, and inability to accurately quantify the fibrosis. Here we propose a fixed time isotropic imaging of the left atrium with a resolution of 1.25mm3. We use retrospective respiratory navigation to remove inconsistent data due to motion and use a constrained reconstruction framework with total variation and 3D block-matching regularizers to remove the data undersampling artifacts. Promising results showing gains from the isotropic acquisitions are presented in canine and human studies.
Introduction
Late Gadolinium Enhancement (LGE) MRI is the gold standard for identifying left ventricular (LV) scar and fibrosis. 3D LGE imaging can be used to detect scar in the left atrium (LA) from catheter ablation [1] in atrial fibrillation (AFIB) patients. LA fibrosis can also be quantified using LGE images [2] and has shown to be a promising biomarker to triage AFIB patients for catheter ablation [3]. LA fibrosis has been related to worse outcomes in patients undergoing ablation [4]. Recently completed Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF2) trial [5] showed benefit in targeting fibrotic regions for ablation in patients with <20% fibrosis. However, in most of these studies [2-4] 15-30% of the images were excluded from fibrosis analysis due to poor quality.3D LA LGE images are acquired with ECG and respiratory navigator gating and typically with anisotropic resolution. Long and variable scan times can result depending on the breathing pattern of the patient and can cause the navigator window to shift, leading to motion artifacts in the images. Long acquisition times can also cause changes in the optimal inversion time for nulling of the myocardium. Obtaining high-quality images rapidly without motion artifacts and with isotropic resolution is crucial for accurate and repeatable quantification of fibrosis. Isotropic acquisitions potentially allow for more accurate quantification of fibrosis from different image orientations. Isotropic 3D LGE acquisitions have been proposed for imaging the LV and LA [6, 7]. Images with 1.4 mm3 resolution were acquired using a diaphragm navigator with an adaptively changing acquisition window [6]. 1.3 mm3 images using an image-based navigator for motion correction were acquired in [7]. Both of these methods use data undersampling and constrained reconstructions to reduce the scan time. Here we propose a fixed time LGE imaging that acquires data irrespective of the navigator position, with a 1.25 mm3 resolution. We use k-means clustering of the navigator signal to identify data corresponding to a consistent motion state and use a combination of total variation [8] and 3D block-matching [9] constraints to remove the artifacts from data undersampling.
Methods
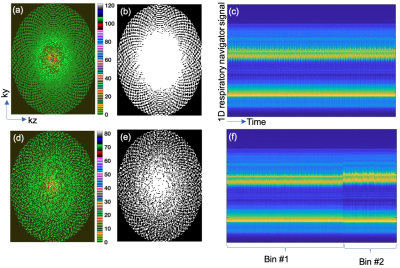
Data acquisition: LGE LA data with isotropic 1.25 mm3 resolution were acquired on a Siemens 3T Prisma scanner in canine and patient studies. The scan parameters were TR=3.3 msec, TE=1.5 msec, flip angle=14o,in-plane FOV=200x320 mm2, in-plane matrix size=160x256. A variable density sampling pattern with golden ratio based angular increments [10] in ky-kz shown in Figure 1a was used. The central ky-kz region is strongly oversampled so that for any given subset of data the contiguous dense sampling in the center of ky-kz is preserved while pseudo-randomly covering the rest of ky-kz as shown in Figures 1d and 1e. Segmented k-space data were continuously acquired in the diastolic phase irrespective of respiratory navigator position for ~8 minutes. One-dimensional (1D) navigator data were acquired after each k-space segment for retrospective navigation. For comparison, we also acquired the standard 3D LGE with the typical anisotropic resolution of 1.25x1.25x2.5 mm3 and with a GRAPPA [11] parallel imaging factor of two and prospective respiratory navigation. The anisotropic acquisitions took ~6 minutes.Reconstruction: Retrospective respiratory navigation of the isotropic data was performed using k-means clustering of the 1D navigator signals into two respiratory bins. Figures 1c and 1f show the navigator signals before and after clustering respectively. Consistent data from the first bin is used for the reconstruction and the data from the second bin is discarded. The corresponding undersampling mask for the first bin is shown in Figure 1d.
Constrained reconstruction of the undersampled data was performed by minimizing Equation (1):
$$$C=‖Am-d‖_2^2+α_1 R_1 (m)+α_2 R_2 (m) -(1)$$$
In the above equation, $$$d$$$ is the retrospectively navigated k-space data, $$$m$$$ is the 3D image estimate, $$$A$$$ is the forward modeling operator that includes the data undersampling pattern and coil sensitivities. The regularizers $$$R_1(m)$$$ and $$$R_2(m)$$$ correspond to total variation (TV) [8] and block matching with 3D denoising (BM3D) [9] constraints respectively with $$$α_1$$$ and $$$α_2$$$ as the corresponding regularization parameters, determined using retrospective undersampling of post-mortem canine data. The combination of the two regularizers removed the undersampling artifacts without making the images blocky. The reconstruction is implemented as decoupled iterations alternating between the TV and BM3D denoising and the data fidelity steps similar to the approach used in [12]. The regularizers are applied on y-z images for each readout. Reconstruction of the anisotropic acquisitions was performed using the scanner’s GRAPPA method [11].
Results
Figure 2 shows the results in different orientations for an invivo canine study. Improved sharpness in the isotropic fixed time acquisition over the anisotropic acquisition can be seen in sagittal and coronal orientations. Figure 3 shows corresponding results from a human subject study.Discussion and Conclusion
With a fixed time acquisition, varying amounts of data may be available for different subjects depending on their respiration patterns and heart rates and rhythms. We found that the reconstruction was robust to modest variations in the amount of data and can still preserve the gains from the isotropic acquisitions. Additional animal and human subject studies are needed to further validate the proposed framework in terms of image quality gains and reliability of fibrosis quantification.Acknowledgements
No acknowledgement found.References
[1] C.J. McGann, E.G. Kholmovski, R.S. Oakes, J.J.E. Blauer, M. Daccarett, et al., New Magnetic Resonance Imaging-Based Method for Defining the Extent of Left Atrial Wall Injury After the Ablation of Atrial Fibrillation, J Am Coll Cardiol, 52 (2008) 1263-1271.
[2] R.S. Oakes, T.J. Badger, E.G. Kholmovski, N. Akoum, N.S. Burgon, et al., Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation., Circulation, 119 (2009) 1758-1767.
[3] N. Akoum, M. Daccarett, C. McGann, N. Segerson, G. Vergara, et al., Atrial Fibrosis Helps Select the Appropriate Patient and Strategy in Catheter Ablation of Atrial Fibrillation: A DE-MRI Guided Approach, Journal of Cardiovascular Electrophysiology, (2010) no-no.
[4] N.F. Marrouche, D. Wilber, G. Hindricks, P. Jais, N. Akoum, et al., Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study, JAMA, 311 (2014) 498-506.
[5] N.F. Marrouche, T. Greene, J.M. Dean, E.G. Kholmovski, L.M. Boer, et al., Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: The DECAAF II trial: Study design, J Cardiovasc Electrophysiol, 32 (2021) 916-924.
[6] T.A. Basha, M. Akçakaya, C. Liew, C.W. Tsao, F.N. Delling, et al., Clinical performance of high-resolution late gadolinium enhancement imaging with compressed sensing, Journal of Magnetic Resonance Imaging, 46 (2017) 1829-1838.
[7] C. Munoz, A. Bustin, R. Neji, K.P. Kunze, C. Forman, et al., Motion-corrected 3D whole-heart water-fat high-resolution late gadolinium enhancement cardiovascular magnetic resonance imaging, Journal of Cardiovascular Magnetic Resonance, 22 (2020) 53.
[8] L.I. Rudin, S. Osher, E. Fatemi, Nonlinear total variation based noise removal algorithms., Physica D, 60 (1992) 259 –268.
[9] K. Dabov, A. Foi, V. Katkovnik, K. Egiazarian, Image Denoising by Sparse 3-D Transform-Domain Collaborative Filtering, IEEE Transactions on Image Processing, 16 (2007) 2080-2095.
[10] S. Winkelmann, T. Schaeffter, T. Koehler, H. Eggers, O. Doessel, An optimal radial profile order based on the Golden Ratio for time-resolved MRI, IEEE transactions on medical imaging, 26 (2007) 68-76.
[11] M.A. Griswold, P.M. Jakob, R.M. Heidemann, M. Nittka, V. Jellus, et al., Generalized autocalibrating partially parallel acquisitions (GRAPPA), Magnetic Resonance in Medicine, 47 (2002) 1202-1210.
[12] E.M. Eksioglu, Decoupled Algorithm for MRI Reconstruction Using Nonlocal Block Matching Model: BM3D-MRI, Journal of Mathematical Imaging and Vision, 56 (2016) 430-440.
Figures