1648
A Cross-Species Approach for Noninvasive in Vivo Tracking of Neutrophil Dynamics upon Cardiovascular Injury by 1H/19F MRI1Heinrich Heine University, Düsseldorf, Germany
Synopsis
Intravenous 19F tracer application for in vivo labelling of neutrophils prior to injury allowed the non-invasive 3D visualization of neutrophils within their different hematopoietic niches over the entire body and the subsequent monitoring of their egress into affected tissues. Stimulated murine/human neutrophils exhibited enhanced labelling which could be exploited as an in vivo readout for their activation state in both sterile and nonsterile cardiovascular inflammation. In summary, the present study demonstrates that both human and murine neutrophils can be specifically targeted to track their dynamic trafficking by non-invasive 1H/19F MRI in vivo.
Introduction
Neutrophils play a complex role during onset of tissue injury as well as subsequent resolution and healing. To more precisely assess neutrophil dynamics upon cardiovascular injury, this study aimed at developing a noninvasive, background-free approach for specific mapping of murine and human neutrophils dynamics by whole-body MRI. For this, targeted multimodal fluorine-loaded nanotracers were engineered with binding peptides specifically directed against murine and human neutrophils, respectively.Methods
Peptides against murine (mNP)[1] and human (hNP)[2] neutrophils as well as corresponding control peptides (Con) were equipped with n-terminal carboxyfluorescein and c-terminal GGG-cysteine spacer, with the latter used for coupling of the peptides to preformed maleimide-PFCs. Binding studies were performed by flow cytometry and 19F MRI using human neutrophils from healthy volunteers and patients with ST-elevated myocardial infarction. Murine neutrophils were obtained from blood of C57BL/6 mice, isolated from the bone marrow, or LPS-doped matrigel plugs[3]. Murine myocardial infarction was induced by 50 min of transient ligation of the left anterior descending artery. MRI experiments were performed at a 9.4T Bruker AVANCEIII Wide Bore NMR spectrometer and datasets were acquired using a 25 mm birdcage resonator tuneable to 1H and 19F.Results and Discussion
Binding studies of mNPPFCs and hNPPFCs revealed the specific labeling of neutrophils from both mice and humans, exhibiting much lower affinity for monocytes or lymphocytes (Figs. 1+2). Moreover, PFCs modified with control peptides (Con) did not show any labelling of neutrophils (Figs. 1+2). These findings were further confirmed by 19F MRI which revealed a stronger 19F signal in neutrophils incubated with NPPFCs as compared to ConPFCs (Fig. 2).To assess neutrophil dynamics upon cardiovascular injury, we used a model of cardiac ischemia/reperfusion, well known to be associated with a massive neutrophil recruitment into the injured myocardium[4]. For monitoring the fate of neutrophils upon myocardial infarction (MI), mice received daily intravenous injections of mNPPFCs over 3 d prior surgery. Whole-body 1H/19F MRI before induction of MI corroborated that this labeling protocol resulted in a strong 19F uptake by bone marrow neutrophils, particularly in femur and tibia (Fig. 3A left). Re-investigation 24 hrs after MI revealed a substantial reduction of 19F signals in these compartments (Fig. 3A, right) with concomitant appearance of 19F label in the infarcted heart. In line with this, flow cytometry identified the femur as main neutrophil reservoir and also as the bone marrow compartment with the largest decrease in neutrophils 24 hrs after MI (Fig. 3B). Focal scanning of the thighs before and 24 hrs after MI confirmed the strong decrease of 19F signals in the femur after MI (Fig. 4A+B). In parallel, images of the thorax unequivocally corroborated the simultaneous appearance of 19F label in the heart (Fig. 4C+D). Cine MRI in combination with late gadolinium enhancement (LGE) demonstrated that the detected 19F pattern perfectly matched the LGE-delineated myocardium (Fig. 4C, top). Importantly, animals which received ConPFCs exhibited significantly less labeling of the bone marrow before MI, which was unchanged after MI and led to only minor amounts of 19F label in the infarcted region (Fig. 4A-D). Furthermore, application of neutralizing antibodies (NAbs) to inhibit the egress of mNPPFC-loaded neutrophils from the femur into the blood blunted the MI-induced effects in heart and femur (Fig. 4C+D).
Next, we verified whether (i) this approach might be transferred to the human setting and (ii) NPPFC uptake by neutrophils is altered under pathological challenges. To mirror the conditions in the murine trafficking experiments after MI, we utilized isolated neutrophils from blood of STEMI patients (i.e. ST elevation MI = STEMI) obtained within the first 24 hrs after MI and found a substantially stronger cellular uptake of hNPPFCs compared to healthy volunteers (Fig. 5A+B). To evaluate whether this observation is associated with various stimuli, we further induced murine inflammation by subcutaneous implantation of a matrigel plug doped with LPS[3]. Both ex vivo incubation of isolated neutrophils and in vivo application of mNPPFCs revealed a more rapid and potent uptake of mNPPFCs by murine neutrophils under stimulated conditions (Fig. 5C+D). Importantly, this effect was restricted to neutrophils while lymphocytes and monocytes showed only minor mNPPFC uptake under inflammatory conditions.
Finally, we investigated whether the enhanced incorporation of mNPPFCs can be exploited to assess in vivo the inflammatory state of neutrophils. To this end, we monitored in situ 19F incorporation into bone marrow neutrophils under stimulated conditions employing again the matrigel/LPS-based inflammation model. Twenty-four hrs after plug implantation, mNPPFCs were applied and further 24 hrs later mice were subjected to 1H/19F MRI. As shown in Fig. 5E-G, we observed a substantially stronger 19F signal in the bone marrow upon LPS pre-activation. This effect became even more evident when relating the detected 19F signal to the number of neutrophils present in the bone marrow (Fig. 5G).
Conclusions
In summary, the present study demonstrates that both human and murine neutrophils can be specifically targeted to track their dynamic trafficking by non-invasive MRI in vivo. In clinical translation, this approach will allow not only to identify hidden origins of bacterial or sterile inflammation in patients but also to unravel cardiovascular disease states that are on the verge of severe aggravation due to enhanced neutrophil infiltration or activation.Acknowledgements
No acknowledgement found.References
- Miettinen, H. M., Gripentrog, J. M., Lord, C. I. & Nagy, J. O. CD177-mediated nanoparticle targeting of human and mouse neutrophils. PLoS ONE 13, e0200444 (2018).
- Mazzucchelli, L. et al. Cell-specific peptide binding by human neutrophils. Blood 93, 1738–1748 (1999).
- Temme, S. et al. Technical advance: Monitoring the trafficking of neutrophil granulocytes and monocytes during the course of tissue inflammation by noninvasive 19F MRI. J. Leukoc. Biol. 95, 689–697 (2014).
- Horckmans, M. et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 38, 187–197 (2017).
Figures
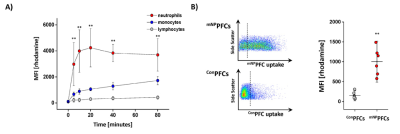
Fig. 1: Specific targeting of murine neutrophils by mNPPFCs
A) Flow cytometric analysis of murine blood leukocytes incubated with mNPPFCs over 80 min illustrating their predominant uptake by neutrophils (red) as compared to monocytes (blue) and lymphocytes (grey). B) Labelling of bone marrow neutrophils isolated 2 hrs upon intravenous injection of mNPPFCs or ConPFCs as determined via flow cytometry indicating a strong uptake of mNPPFCs and almost no incorporation of ConPFCs.
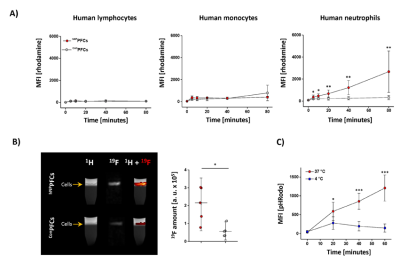
Fig. 2: Specific targeting of human neutrophils by hNPPFCs
A) Uptake of hNPPFCs (red) or ConPFCs (grey) by human lymphocytes, monocytes or neutrophils as determined by flow cytometry. B) For MRI analysis, human immune cells were incubated with hNPPFCs (upper row) or ConPFCs (lower row). Cells were purified by density gradient centrifugation and analyzed by MRI. A quantification of the 19F data is shown on the right. C) hNP was conjugated to the pH-sensitive dye pHRodo and incubated with neutrophils at 4 °C (blue) and 37 °C (red) demonstrating energy dependent uptake of the particles.

Fig. 3: Whole-body 3D 1H/19F MRI of murine neutrophils by mNPPFCs in vivo
A) Anatomical 1H data were rendered transparent in gray with 19F data overlayed in orange/red; for the sake of clarity signals from the liver/spleen were faded out. Left: Application of mNPPFCs prior MI resulted in in situ labeling of neutrophils within their hematopoietic niches. Right: 24 hrs post MI - pronounced reduction of 19F signals in the bone marrow of femur and tibia with simultaneous appearance of 19F label in the infarcted heart. B) Post mortem flow cytometry of the different bone marrow compartments.
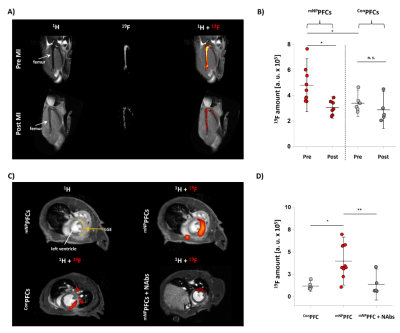
Fig. 4: Mapping the trafficking of murine neutrophils after MI by mNPPFCs in vivo
A) 1H/19F MRI of the bone marrow after in vivo labelling with mNPPFCs/ConPFCs. B) Quantification demonstrated significantly reduced 19F femur signals post MI with mNPPFCs. C) Thoracal 1H/19F MRI revealed distinct 19F signals in the infarcted region with mNPPFCs. Substantially lower 19F deposition was observed within infarcted myocardium with ConPFCs/mNPPFCs or when neutrophil egress was inhibited by neutralizing antibodies (NAbs). D) Quantification of the cardiac 19F MR signal for all treatments.
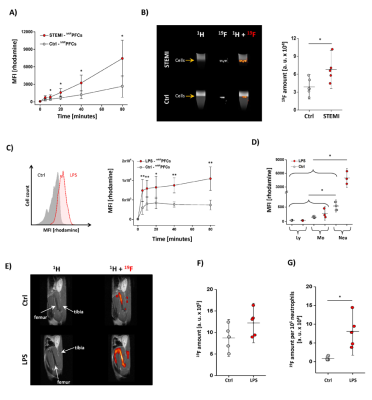
Fig. 5: Inflammatory stimuli increase uptake of NPPFCs by neutrophils
hNPPFC uptake by neutrophils from STEMI patients and healthy controls by flow cytometry (A) and 1H/19F MRI (B). C) Ex vivo uptake of mNPPFCs by neutrophils from mice with a matrigel/LPS plug (red) vs. control (grey). D) In vivo uptake of mNPPFCs by immune cells of mice with a matrigel/LPS plug. E) mNPPFC injection into mice with matrigel/LPS and 1H/19F MRI of the bone marrow after 24 hrs. D) Quantification of the total amount of 19F in the bone marrow and E) normalization of the 19F signal to the total number of neutrophils.