1646
Trajectory correction enables Free running cardiac DIXON at 3T1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, Lausanne, Switzerland, 5Center for Biomedical Imaging, Lausanne, Switzerland, 6Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 7Siemens Healthcare, MR Application Development, Erlangen, Germany, 8Department of Diagnostic, Interventional and Pediatric Radiology, Bern University Hospital (Inselspital), Bern, Switzerland, 9Translational Imaging Center, sitem-insel, Bern, Switzerland
Synopsis
Free-running cardiac DIXON-MRI has potential for T2*/PDFF quantification to explore cardiac fat accumulation and alteration in metabolic diseases. DIXON at 3T suffers from rapid fat-water phase accrual and inhomogeneous B0. Consequently, bipolar echoes, as opposed to monopolar, are required to achieve equal/shorter than in-phase/out-of-phase echo spacing. However, distortions occur between even and odd echoes due to gradients imperfections. Thus, the existing framework was extended with a k-space trajectory correction based on gradient impulse response function(GIRF). Both monopolar or bipolar echoes without GIRF correction resulted in fat-water swaps and unreliable quantitative maps, that were resolved using GIRF-corrected bipolar free-running DIXON.
Background
There is a growing interest in probing cardiac fat which has been shown to influence pathophysiological pathways towards cardiovascular degradation in metabolic diseases1. Chemical shift-encoded (CSE) MRI enables high resolution mapping of proton density fat-fraction (PDFF), non-invasive biomarker of fatty depots2 as well as the quantification of T2* decay, which could further probe iron overload and haemorrhage. Nevertheless, CSE-MRI or DIXON-MRI, while benefiting from increased field-strength at 3T, remains challenging due to rapid phase accrual between water and fat concomitant with inhomogeneous B03 in the heart. The free-running framework is a novel approach for high-resolution cardiac imaging with fully respiratory and cardiac self-gating, combined with a multidimensional Compressed Sensing reconstruction4. We hypothesized that the free-running framework lays ground for high-resolution cardiac DIXON-MRI at 3T. Only bipolar multi-echoes achieve short-enough echo spacing at 3T but suffer from distortions between odd and even echoes due to gradient imperfections. Thus, free-running DIXON was extended with k-space trajectory correction using gradient impulse response function (GIRF), which enabled high-resolution cardiac DIXON at 3T.Methods
A custom-written prototype 3D radial spoiled gradient echo sequence was implemented with multiple echoes and a phyllotaxis trajectory for integration with the free-running framework4. Data were acquired on a 3T scanner (Vida, Siemens) with the spine coil and an 18-channel body coil.With a TR of 15ms, which is sufficient to quantify fatty acid composition (FAC) parameters5 and T2* decay, 13 echoes (TE1/ΔTE = 1.12/1.07ms) in bipolar mode and 8 echoes (TE1/ΔTE = 1.16/1.96ms) in monopolar mode were obtained. For a ten minutes acquisition, 40014 radial views per echo (13 segments, 3078 Shots) were acquired with FOV=220mm at isotropic 1.5mm resolution, FA=5°, BW=1510 Hz/px for all echoes.
The system-specific Gradient system Impulse Response Function (GIRF) was measured using the 2-offcentered slices method on a spherical phantom at isocenter6,7. The gradients (Fig1) were corrected using multiplication in the frequency domain between the GIRF and the DIXON free-running gradients waveforms, and provided the actual k-space trajectory.
Cardiac and respiratory motion signals were extracted from the first echo of the superior-inferior projections4. Data were binned in 4 respiratory phases and 100ms-wide cardiac phases. The 6D binned k-space data were reconstructed using the free-running compressed sensing framework4. Based on 3D radial Nyquist criteria, individual free-running DIXON data were accelerated by a factor R=26 for 8 cardiac phases and 4 respiratory state.
Complex images from each bin were processed for fat-water separation using Iterative Decomposition of water and fat with Echo Asymmetry and Least square Estimation (IDEAL) method with constrained extension8. Performances of the proposed correction were evaluated on a phantom, on in vivo images and quantitative maps (PDFF, R2*).
Results
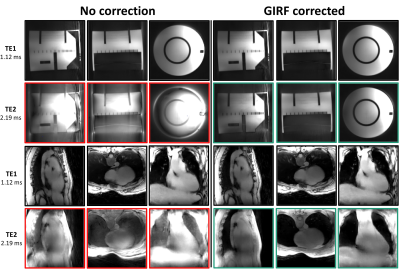
The GIRF correction revealed subtle oscillations on gradient waveforms and roughly a shift of one sampled point in k-space trajectory on even echoes(Fig1). Thus, strong radial artifacts appeared between odd and even echoes images that were resolved with GIRF correction(Fig2). Odd echoes were not impacted thanks to a sequence-level calibration for the 1st echo and balanced errors between odd and even gradients.At 3T, despite a high bandwidth and modest matrix size, monopolar echoes were unable to reach an echo spacing shorter than the required in-phase/out-of-phase 1.24ms delay (black markers in Fig. 3). Only bipolar echoes achieved short-enough echo spacing (ΔTE=1.07ms). Additionally, within TR=15ms, 13 bipolar echoes were acquired against 8 monopolar echoes only(Fig3).
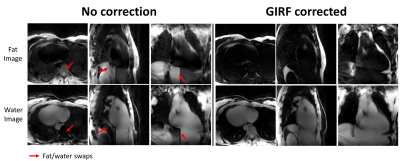
Without GIRF correction, fat/water images were incorrectly reconstructed (Fig4) with blurring at the apex and around the atria and massive swaps between fat and water. On the contrary, GIRF corrected images were sharper and fat/water swaps were not observed.
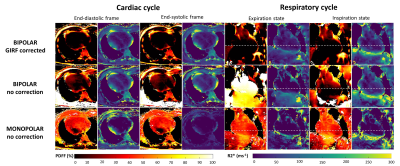
Whether in monopolar or in bipolar mode without correction, PDFF and T2* maps were highly overestimated across the cardiac and respiratory cycles (Fig5). In end-diastolic images, mean PDFF in the heart was 36%, 11% and more realistically 2% for monopolar mode, bipolar without correction and for GIRF corrected bipolar respectively. This overestimation of fat was visible at all motion states. Similarly, recurrent underestimations were observed in R2* maps.
Discussion
Aiming for high-resolution cardiac fat and water imaging, free-running cardiac DIXON proved to require bipolar echoes at 3T. However, gradient imperfections hampering the demanding high gradient throughput, a correction with the system-specific GIRF was required to prevent radial-trajectory image artifacts. Eventually, GIRF-corrected free-running provided fat/water swaps-free motion-resolved volumes, paired with PDFF and T2* maps. Of note, quantitative PDFF and T2* maps were also corrupted using a graph-cut algorithm9, confirming the image quality were the culprit.Providing full cardiac and respiratory cycles to evaluate cardiac fat in metabolic diseases offers multiple benefits: 1/ to study epicardial fat, systole is preferred when pericardium is thicker, 2/ B0 inhomogeneity tends to be reduced during expiration, limiting local signal loss and phase accumulation and 3/ variations of R2* along the cardiac cycle in the myocardium but also between right and left ventricular blood pools hold interest to probe cardio-respiratory status. Further investigation leveraging the 13 echoes could provide valuable FAC characterization.
Conclusion
This study aims at providing free-running cardiac DIXON-MRI at 3T to enable high resolution PDFF and T2* mapping along the cardiac and respiratory cycle to reliably study cardiac fat in metabolic diseases.Acknowledgements
This project has received financial support from the CNRS through a MITI program and was performed within a laboratory member of France Life Imaging network. (grant ANR-11-INBS-0006)References
1. Gaborit, B., Sengenes, C., Ancel, P., Jacquier, A. & Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? in Comprehensive Physiology (ed. Terjung, R.) 1051–1082 (John Wiley & Sons, Inc., 2017). doi:10.1002/cphy.c160034.
2. Reeder, S. B., Hu, H. H. & Sirlin, C. B. Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging 36, 1011–1014 (2012).
3. Rajiah, P. & Bolen, M. A. Cardiovascular MR Imaging at 3 T: Opportunities, Challenges, and Solutions. RadioGraphics 34, 1612–1635 (2014).
4. Di Sopra, L., Piccini, D., Coppo, S., Stuber, M. & Yerly, J. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med 82, 2118–2132 (2019).
5. Schneider, M. et al. Accurate fatty acid composition estimation of adipose tissue in the abdomen based on bipolar multi‐echo MRI. Magn. Reson. Med. 81, 2330–2346 (2019).
6. Campbell-Washburn, A. E., Xue, H., Lederman, R. J., Faranesh, A. Z. & Hansen, M. S. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function: Real-Time Distortion Correction Framework for Fast Imaging. Magn. Reson. Med. 75, 2278–2285 (2016).
7. Berzl,M.,Pfeuffer,J. et al. Improved spiral trajectory correction using the gradient impulse response function (GIRF). ISMRM 2017,933.
8. Bydder, M. et al. Constraints in estimating the proton density fat fraction. Magnetic Resonance Imaging 66, 1–8 (2020).
9. Andersson, J., Ahlström, H. & Kullberg, J. Water-fat separation incorporating spatial smoothing is robust to noise. Magnetic Resonance Imaging 50, 78–83 (2018).
Figures