1634
Associations of Aging- and AD-associated 7T MRS Neurochemical Profiles with Cognition and Polygenic Risk for AD1University of Minnesota, Minneapolis, MN, United States, 2Washington University School of Medicine, St. Louis, MO, United States, 3Minneapolis VA Health Care System, Minneapolis, MN, United States, 4University of Oxford, Oxford, United Kingdom, 5University of Missouri, Columbia, MO, United States
Synopsis
Ultra-high field (7T) ultra-short echo time (8 ms STEAM) MRS can consistently provide a 14-neurochemical profile and distinguish aging and AD status. In this project, we generated composite scores from published age- and AD- specific neurochemical profiles and applied them to a typically aging cohort for whom polygenic risk for AD and extensive cognitive performance data were also available. Principal component analysis of the neurochemical profile fully distinguished aging, and AD classification was 58% correct. The largest correlation coefficients (r) were found between MRS and cognition. Correlation between MRS and polygenic risk and between cognition and polygenic risk was smaller.
Introduction
7T MRS allows for quantification of a 14-neurochemical profile of the human posterior cingulate cortex (PCC), which is known to deteriorate in Alzheimer’s disease (AD). We recently reported 5 age-associated differences in the PCC of participants in age ranges 18-22 versus 70-89 years (Marjanska 2017). We also reported early stage AD-associated differences in 3 neurochemical concentrations in the PCC, and the neurochemical profile was able to distinguish participants with AD from controls with same-sample sensitivity 88% and specificity 97% (Marjanska 2019). In this project we generated composite scores from these published neurochemical profiles and applied them to a typically aging cohort spanning ages 35-89 for whom polygenic risk for AD and extensive cognitive performance data were also available. The goals were to investigate whether MRS-based indices of aging and AD correlate with cognitive performance and/ or AD risk, and which of MRS or genetic risk correlate best with cognitive performance.Methods
14-neurochemical profiles were measured from the PCC of 45 typically aging adults aged 36-88 (table 1) who did not show signs of incipient AD when examined by the study neurologist. These participants underwent ultra-short echo time (8 ms STEAM) single voxel (8 cm3) 7T MRS of the PCC which was quantified using LCModel with 18 neurochemicals in the basis set, measured macromolecule spectra, and basis spectra for falx cerebri lipids and methylsulfonylmethane as in the published study (Marjanska 2019). To avoid over-informing the model, the MM spectrum from young participants was used, and a basis spectrum was added to account for age-associated differences in the MM spectrum at 1.7 ppm (Marjanska et al 2018). Concentrations were quantified relative to total Creatine to avoid informing PCA of age via age-associated differences in water content (i.e., as would arise if water were used as an internal reference). Unsupervised principal component analysis (PCA) was conducted (JMP software) on the pure aging and AD cohorts from the publications, then these principal components were calculated for the new typically aging cohort. These principal components were also computed across the aging and AD datasets. Polygenic risk for AD was calculated with and without including APOE4 status in the classifier (Cruchaga et al 2018). Cognitive performance was measured using the procedures of the human connectome project on aging (Bookheimer et al 2019) and composite scores for memory and executive function were constructed using factor analysis on 25 behavioral variables on 532 subjects age 35-60. These cognitive composites have an age dependence, as expected.Results
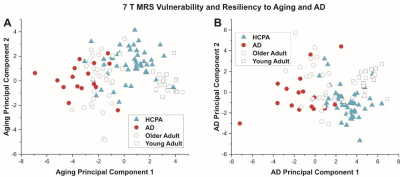
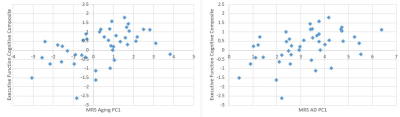
Figure 1 shows the PCA results. PCA fully separated the young and older adult age groups by the first principal component (PC1). For AD, the classification was 58% correct, and much of the separation was apparent in PC1. The overlay of AD data on the PCA for aging suggested that AD tends to be an extension of aging, and the overlay of aging data on PCA for AD shows that no young adults (age 19-22) had brain chemistry comparable to participants with AD. MRS quality for the HCP-A cohort was comparable to that in the publications on aging and AD (Marjanska et al 2017 and 2019). Correlations among PC1 for each of aging and AD, the cognitive indices for memory and executive function, and the polygenic risk scores (with and without APOE4 status included) are shown in table 2 and the largest correlations are illustrated in figure 2. Correlation coefficients (r) ≥ 0.4 were found between MRS and cognition, whereas correlation coefficients between MRS and polygenic risk were low (r ≤ 0.3), as were correlations between cognition and polygenic risk (r < 0.2).Discussion
The main observation of this study is that neurochemical profiles have larger correlations coefficients with cognition than genetics, and neurochemical profiles have larger correlation with cognition than genetics have with cognition. Another surprising observation is that although unsupervised MRS-based separation of AD from control was marginally successful, the first principal component for AD was strongly associated with cognition in typically aging non-demented adults. Whereas it is possible that age moderates the relationship between the MRS aging marker and cognition, the MRS AD marker pays no respect to age. The MRS aging marker also correlated (r=0.36) with the fully normed picture sequence memory test scores, which remove age as a cofactor.Conclusions
MRS is remarkably sensitive to cognitive function. It provides new insight on neurodegenerative processes underlying cognitive decline, since it is not based on morphometric features or hallmarks of AD. MRS may contribute new knowledge on factors that impact cognitive function. It is also suitable to monitor treatment response.Acknowledgements
Cognitive and Genetic data obtained from the Human Connectome Project on Aging, www.humanconnectome.org/study/hcp-lifespan-aging
MRS data from NIH R01 AG 055591
References
Marjanska et al Neuroscience 354 (2017) 168–177
Majanska et al Journal of Alzheimer’s Disease 68 (2019) 559–569
Marjanska et al NMR Biomed. 31(2) (2018)
Cruchaga et al Alzheimers Dement. 14 (2018) 205-214
Bookheimer et al NeuroImage 185 (2019) 335–348
Figures