1633
Age-related decline of glutathione: a magnetic resonance spectroscopy study in 180 elderly participants1Department of Neuroradiology, University Hospital Zurich, Zurich, Switzerland, 2Department of Nuclear Medicine, University Hospital Zurich, Zurich, Switzerland, 3Institute of Regenerative Medicine Zurich, University of Zurich, Zurich, Switzerland, 4Institute of Regenerative Medicine Zurich., University of Zurich, Zurich, Switzerland, 5Center for MR Research, University Children's Hospital, Zurich, Switzerland
Synopsis
Glutathione (GSH) is a brain marker for oxidative stress, which has previously been associated with brain amyloidosis and memory decline. However, to date no study has examined the link between GSH and beta-amyloid in a large cohort of elderly participants. Using simultaneous PET/MRS, we assessed amyloid deposition with amyloid-PET and GSH with MEGAPRESS, in a cohort of 134 healthy elderly and 46 mild cognitively impaired participants. GSH declined with age and showed sex differences, but no significant association between GSH and amyloid status was observed
Introduction
The antioxidant glutathione (GSH) is a brain marker of oxidative stress reserve and a key “hunter” for cellular free radicals. So far, few studies analysed glutathione in patient populations1-4 (including Alzheimer disease (AD)5) or in in cognitively healthy elderly6. Only one study assessed whether GSH levels, detected noninvasively with proton MR spectroscopy (MRS), were associated with brain amyloidosis and memory in 15 healthy older adults7. The authors found that GSH levels were significantly associated with greater brain amyloidosis in the temporal and parietal regions, adjusted for Apolipoprotein E epsilon4 (APOE4) carrier status. Yet, no study has yet examined GSH concentrations and its link to beta-amyloid (β-amyloid) within the posterior cingulate cortex (PCC) in a larger cohort of cognitively healthy elderly, although the PCC demonstrates functional and metabolic (i.e. neurotransmitter) alterations in healthy cognitively older adults8 and in individuals with mild to severe cognitive impairments9-11. We hypothesized that GSH is associated with age and β-amyloid, i.e. that GSH declines with age and that this decline is steeper in patients with higher β-amyloid load.Methods
In a final sample of 134 healthy controls (HC) and 46 individuals with mild cognitive impairment (MCI), simultaneous PET and MRI/MRS data were acquired on a 3 Tesla GE PET/MR scanner equipped with an 8-channel head coil. MRS data were collected with MEGA-PRESS12 (TE/TR = 131/1800 ms, editing frequencies: 4.56/20 ppm). A 3D T1-weighted spoiled gradient echo (SPGR) volume was also acquired for partial volume correction of the GSH concentrations. Simultaneous PET data were collected dynamically 80 minutes after tracer injection of 140 MBq 18F-Flutemetamol (matrix 256*256*89 and voxel size: 1.172*1.172*2.78 mm), and the β-amyloid deposition of the PCC was determined on the averaged frames from 85–110 minutes post injection using PMOD 3.9 and a grey matter voxel mask. As reference, cerebellum grey matter values (from the Hammers Atlas) were used. Centiloid values were calculated with PMOD 4.1. The centiloid cut-off value for amyloid status was set to 30, resulting in 156 β-amyloid negative and 24 positive cases. We also recorded the APOE4 status and classified subjects into E4 carrier (41 total, 33 HC) and E4 non-carrier (139 total, 101 HC). MRS data were pre-processed in Gannet and analysed in LCModel13. The GSH/NAA ratios from the edited spectra were multiplied by the water-scaled NAA concentrations (derived from the edit OFF lines), and the resulting GSH concentrations were corrected for relaxation14 and partial volume effects. For the statistical analysis, a Spearman analysis was performed (GSH with age, β-amyloid standardized uptake value ratio, voxel-specific grey matter volume (GMV)). Subsequently, a stepwise regression model was used to determine the association of GSH (dependent variable) with amyloid status, sex, APOE4, diagnostic code (HC/MCI), GMV, and age.Results
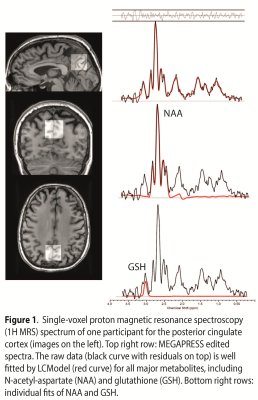
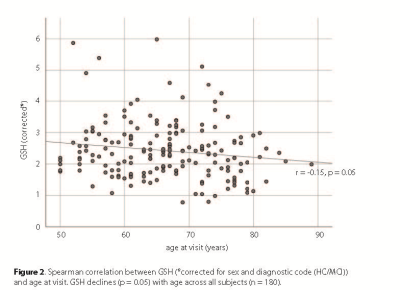
A GSH-edited spectrum is shown in Figure 1, with the spectral fit overlaid. GSH declined significantly with age (r=-0.147, p=0.050, two-tailed, Figure 2), controlling for diagnostic code and sex. There was also an inverse correlation between GSH levels and GMV (r=-0.147, p=0.049, two-tailed). When controlling for GMV, APOE4, and diagnostic code, the GSH*age correlation reduced to trend level (r=-0.143, p=0.059). The regression analysis revealed three significant models (model 3: GMV removed; F(4)=2.43, p=0.050, model 4: β-amyloid load additionally removed; F(3)=2.91, p=0.036, model 5: diagnostic code additionally removed, F(2)=2.96, p=0.025), indicating that age and sex are the strongest predictors of GSH.Discussion
The age-related in GSH in the PCC region suggest a reduced capacity of free radical clearance. Extant data from in vivo animal models and postmortem studies indicate that AD pathology is associated with reduction of GSH15,16. Few studies investigated GSH modulation in the brain with AD and assessed the diagnostic potential of GSH as a biomarker for AD and MCI5. Mandal et al. (2015) reported an AD-dependent reduction of GSH in the bilateral hippocampi and frontal cortices. Moreover, the GSH drop in these regions correlated with decline in cognitive functions. Previously hippocampal GSH was shown to discriminate between MCI and HC with 87.5% sensitivity, 100% specificity, whereas cortical GSH differentiated MCI and AD with 91.7% sensitivity, 100% specificity. In contrast, our study did not show a significant GSH difference between HC and MCI in the PCC, but future studies are needed to confirm if this is true when comparing HC to severely cognitive impaired individuals (e.g. AD). In addition, the presence of significant β-amyloid load does not lead to a steeper decline in GSH with age, as amyloid was unrelated to GSH. Yet, our sample of primarily healthy participants contained only 24 (i.e. 13%) amyloid positive cases and thus, a link of GSH and amyloid (in the PCC) cannot be excluded but might be more relevant in a later stage of neurodegeneration.Conclusion
In a large cohort of healthy elderly and MCI participants, GSH was significantly linked to age and sex but not to amyloid status. However, larger studies in patients with significant b-amyloid deposition may be able to clarify whether GSH can predict further cognitive decline.Acknowledgements
We thank all subjects for their participation in the study.References
1. Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl). 2013 Aug 17.
2. Michels L, Schulte-Vels T, Schick M, et al. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: preliminary findings. Psychiatry Res. 2014 Dec 30;224(3):288-95.
3. An L, Dani KA, Shen J, Warach S, Natural History of Stroke I. Pilot results of in vivo brain glutathione measurements in stroke patients. J Cereb Blood Flow Metab. 2012 Dec;32(12):2118-21. 4. Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging. 2010 Feb;28(2):163-70.
5. Mandal PK, Saharan S, Tripathi M, Murari G. Brain glutathione levels--a novel biomarker for mild cognitive impairment and Alzheimer's disease. Biol Psychiatry. 2015 Nov 15;78(10):702-10.
6. Emir UE, Raatz S, McPherson S, et al. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011 Aug;24(7):888-94.
7. Chiang GC, Mao X, Kang G, et al. Relationships among Cortical Glutathione Levels, Brain Amyloidosis, and Memory in Healthy Older Adults Investigated In Vivo with (1)H-MRS and Pittsburgh Compound-B PET. AJNR Am J Neuroradiol. 2017 Jun;38(6):1130-7.
8. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009 Jul 30;63(2):178-88.
9. Ng S, Villemagne VL, Berlangieri S, et al. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer's disease. J Nucl Med. 2007 Apr;48(4):547-52. 10. Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007 May 8;68(19):1603-6.
11. Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007 May 15;68(20):1718-25.
12. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998 Oct;11(6):266-72.
13. Brix MK, Ersland L, Hugdahl K, et al. Within- and between-session reproducibility of GABA measurements with MR spectroscopy. Journal of Magnetic Resonance Imaging. 2017 Aug;46(2):421-30. 14. Sanaei Nezhad F, Anton A, Parkes LM, Deakin B, Williams SR. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magn Reson Med. 2017 Oct;78(4):1257-66.
15. Ramassamy C, Averill D, Beffert U, et al. Oxidative insults are associated with apolipoprotein E genotype in Alzheimer's disease brain. Neurobiol Dis. 2000 Feb;7(1):23-37. 16. Gu M, Owen AD, Toffa SE, et al. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci. 1998 Jun 11;158(1):24-9.
Figures