1624
Characterizing Heterogeneity of Gliomas with a Fractional-order Calculus Diffusion Model1Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan, China, 2Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
This study aimed to investigate whether the FROC diffusion model could help predict molecular biomarkers in gliomas. It was found that FROC has the potential in comprehensively characterizing heterogeneity of gliomas, not only in glioma grading, but also in predicting tumor cell proliferation rate and isocitrate dehydrogenase-1 (IDH-1) gene mutation status.
Introduction
Gliomas are the most common primary brain tumor in the central nervous system.1,2 Characterizing heterogeneity is important for the accurate diagnosis and treatment planning of gliomas. The fifth edition of World Health Organization classification of tumors in the central nervous system (WHO CNS5) emphasized more on the role of molecular biomarkers in grading and classification of gliomas, especially the IDH-1 mutation.3 The Ki-67 is a key prognostic molecular biomarker for glioma patients, which can reflect the tumor cell proliferation rate.4 Previous studies have demonstrated the potential of FROC parameters in differentiating benign and malignant tumors.5,6 However, as far as we know, the utility of the FROC diffusion model for detecting IDH-1 mutation status and Ki-67 index of gliomas has not been reported yet.Methods
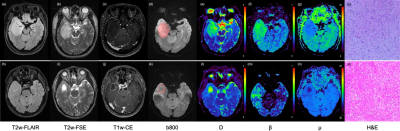
This prospective study was approved by the local ethics committee. A total of 45 patients (23–78 years of age) with pathologically confirmed gliomas were recruited, including 14 IDH-1 mutation gliomas and 31 IDH-1 wild gliomas. MR scans were performed on a clinical 3.0T scanner (uMR 790, United Imaging Healthcare, Shanghai, China) with a commercial 24-channel phased-array head-neck coil. The scanning protocols included single-shot diffusion echo-planar imaging with 12 b-values (b-values=0, 20, 40, 60, 80, 200, 500, 800, 1500, 2000, 2500, 3000 s/mm2), 3D T2-FLAIR, 2D T2-weighted FSE, and 3D T1-weighted GRE. The anomalous diffusion coefficient (D), intra-voxel diffusion heterogeneity (β) and microstructural quantity (μ) were derived from the FROC diffusion model, and the ADC was derived from a mono-exponential model for comparison. D, β, μ and ADC in the solid part of tumors were compared between the low-grade gliomas (LGG) and high-grade gliomas (HGG), and compared between the IDH-1 mutant type and IDH-1 wild type. The Mann–Whitney U test was performed and receiver operating characteristic (ROC), were used to assess the performance. All statistical analyses were performed in SPSS. The significance threshold was set at 0.05.Results
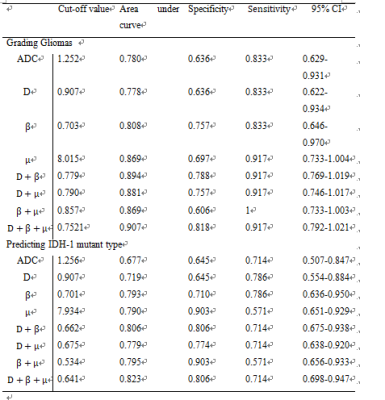
D, β, and ADC were significantly higher in high-grade gliomas and in low-grade gliomas, while μ were significantly lower in high-grade gliomas and in low-grade gliomas. D and β were significantly higher in IDH-1 mutant gliomas than in IDH-1 wild gliomas, while μ were significantly lower in IDH-1 mutant gliomas than in IDH-1 wild gliomas, as showed in Figure 1 and Figure 2. The combination of D, β and μ provided the highest area under the ROC curve (AUC: 0.907) in glioma grading, as well as the highest AUC in identifying IDH-1 mutation status (AUC: 0.823) (as showed in Table 1).Discussion
Significantly higher D, β and ADC in LGGs than in HGGs were agreed with previous findings, which might be due to higher tumor cell cellularity and increased tissue heterogeneity in HGGs than LGGs.5 As μ has a negative correlation with the mean free diffusion length,6 our finding in μ might result from more restricted diffusion of water molecules within the tumor microenvironment of HGGs than LGGs. Our study has also shown the potential of a FROC diffusion model in identifying IDH-1 mutation status.Previous studies have suggested that the IDH-1 mutation might play a role in blocking differentiation of glioma stem cells.7 Besides, the IDH-1 mutation could promote the formation of tumor microvessels by up-regulating vascular endothelial growth factor (VEGF).7 Therefore, our findings in FROC parameters might result from less cellularity and higher vascularity in IDH-1 mutant gliomas than in IDH-1 wild gliomas. Moreover, β and μ outperformed the conventional ADC in predicting Ki-67 index and IDH-1 mutation status, which might be due to FROC parameters β and μ were able to characterize non-Gaussian diffusion of water molecules within tumor, while the conventional ADC can’t.6Conclusion
Our results showed that FROC has the potential in comprehensively characterizing heterogeneity of gliomas, not only in glioma grading, but also in predicting tumor cell proliferation rate and IDH-1 gene mutation status.Acknowledgements
This work is supported by National Key R&D Program of China 2017YFC0108803.References
1. Gittleman H, Boscia A, Ostrom QT, Truitt G, Fritz Y, Kruchko C, Barnholtz-Sloan JS. Survivorship in adults with malignant brain and other central nervous system tumor from 2000-2014. Neuro Oncol 2018;20(suppl_7):vii6-vii16. doi: 10.1093/neuonc/noy090.
2. Alfonso JCL, Talkenberger K, Seifert M, Klink B, Hawkins-Daarud A, Swanson KR, Hatzikirou H, Deutsch A. The biology and mathematical modelling of glioma invasion: a review. J R Soc Interface 2017;14(136):20170490. doi: 10.1098/rsif.2017.0490.
3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106.
4.Chen WJ, He DS, Tang RX, Ren FH, Chen G. Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev 2015;16(2):411-20. doi: 10.7314/apjcp.2015.16.2.411.
5.Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, Zhou XJ. Differentiation of Low- and High-Grade Pediatric Brain Tumors with High b-Value Diffusion-weighted MR Imaging and a Fractional Order Calculus Model. Radiology 2015;277(2):489-96. doi: 10.1148/radiol.2015142156.
6.Chen W, Zhu LN, Dai YM, et al. Differentiation of salivary gland tumor using diffusion-weighted imaging with a fractional order calculus model. Br J Radiol 2020;93(1113):20200052. doi: 10.1259/bjr.20200052.
7.
Huang
J, Yu J, Tu L, et al. Isocitrate Dehydrogenase Mutations in Glioma: From Basic
Discovery to Therapeutics Development. Front Oncol 2019;9:506. doi:
10.3389/fonc.2019.00506.
Figures