1620
Imaging Off-Center: How far can we move outside the central imaging region1Department of Radiology, Medical Physics, University Freiburg, Faculty of Medicin, Freiburg, Germany, 2Department of Radiology, University Freiburg, Faculty of Medicin, Freiburg, Germany, 3Center for Medical Physics and Biomedical Engineering, High Field MR Center, Medical University of Vienna, Vienna, Austria
Synopsis
Imaging outside the predefined and optimized FoV may enhance accessibility for interventional MRI procedures. Here we present different measures to assess the encoding capability of gradient systems in off-center regions. Different clinically establishes sequences are compared at 0.55T and 1.5T regarding their imaging capabilities beyond the predefined target regions.
Introduction
MRI systems are usually designed for a spherical or cylindrical target imaging volume. In this wok we want to explore the feasibility of image acquisition beyond the specified and optimized region. Off-center imaging is especially interesting for interventional procedures where accessibility is important. Here we introduce new measures to assess the encoding capability of the gradient system and of the main magnet. Clinically established MRI sequences are compared in off-center image acquisition at an experimental 0.55T MRI and a clinical 1.5T MRI systems.Methods
For MR imaging three properties are required:- partial orthogonality of three spatial encoding magnetic fields (SEMs) for signal encoding;
- a main magnetic field with a reasonable homogeneity for sample magnetization;
- sufficiently homogeneous B1 field for excitation.
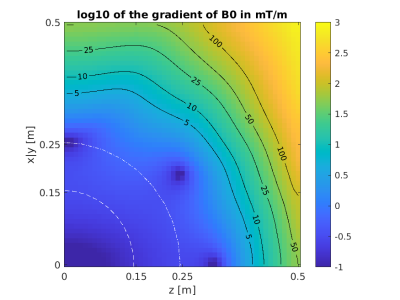
For analyzing the B0 field we chose to look at the gradient of the main field. If the gradient of $$$B_0$$$ is much stronger than the SEMs, imaging is expected to become difficult due to dephasing effects. The resulting deviation of $$$B_0$$$ may prohibit imaging due to bandwidth limits for possible RF-excitations.
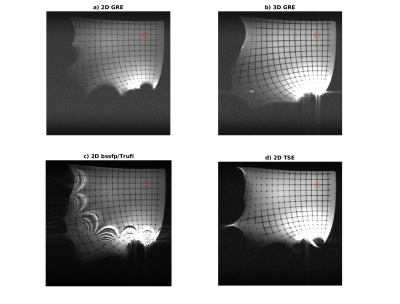
MRI sequences were written in Matlab using the PulSeq [3,4] sequence programming environment. Sequences were optimized to achieve minimum echo time, TE, for the following sequences: 2D gradient echo (2D-GRE) [5], 3D gradient echo (3D-GRE), 2D RARE/TSE [6], 3D RARE/TSE [7], 2D bssfp/Trufi. Imaging experiments were performed on a 1.5T MAGNETOM Aera (Siemens Healthineers, Erlangen, Germany) with XQ Gradients (45 mT/m @ 200 T/m/s) and an experimental 0.55T whole body MRI scanner with an 80cm bore and a gradient system capable of 33 mT/m @ 125 T/m/s. A grid structure with a grid size of 20mm was 3D printed in house to uncover spatial distortions in combination with a large phantom container.
Results
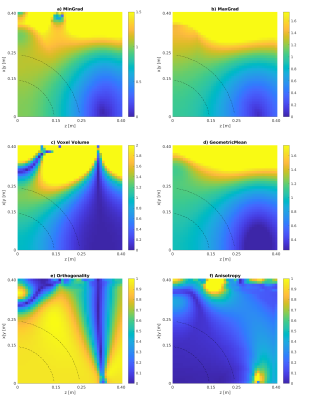
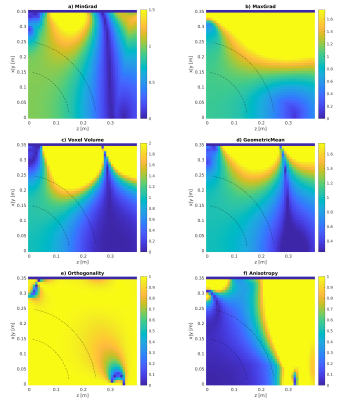
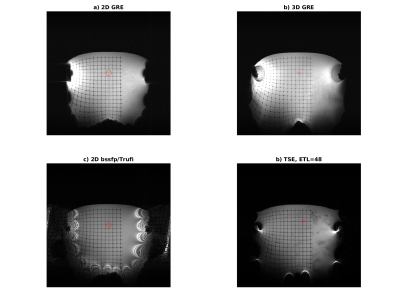
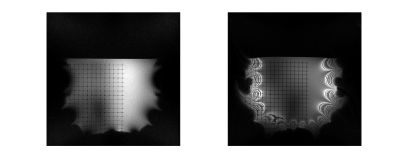
Maps of the different metrics for the encoding fields (dot-cross product, local orthogonality, geometric mean and minimum gradient) are depicted in Fig.1 and Fig.2 for two different gradient systems. A plot of the resulting gradient of the theoretical $$$B_0$$$ field of the 0.55T scanner is shown in Fig.3. Experimental images for different clinically established sequences are depicted in Fig.4 and Fig.5. Distortion corrected images are shown in Fig.6.In general, 3D sequences seem to be beneficial compared to 2D. Additionally, smaller slice thickness enables for enhanced imaging capabilities off-center which can be explained by through-slice intra-voxel dephasing effects. For TSE acquisitions we found that shorter echo-train-lengths enable for acquiring a larger FoV. The available bandwidth of both systems was not limiting image acquisition.
In comparison the 3D GRE sequence and the bssfp/ TrueFISP (despite severe banding artifacts) sequence reach out the furthest. 0.55T magnets seem to have an enhance region for off-center imaging due to less pronounced gradients of the $$$B_0$$$ field.
Discussion
The feasibility of image acquisition off-center, outside the optimized imaging volume, is limited by the available hardware. It appears that a region moving diagonally out from the iso-center might be especially usefull for image encoding. If this his cone shaped volume is taken into account while designing MRI systems an enhanced imaging volume might become available. The main applications which would benefit from imaging in this region are interventional procedures, eg catheter or needle tracking.The feasibility of in vivo imaging will be validated in a next step. In addition advanced sequence concepts should be considered, eg missing pulse bssfp [7,8] or others. Larger bore magnets appear to be very promising for extending the FOV. Such magnets may become more widely accessible with the recently established field strength of 0.55T.
Acknowledgements
The authors would like to thank Axel vom Endt and Andrew Dewdney from Siemens Healthineers for supplying the data and for in depth discussions on gradients and shims. Matthias Malzacher, Manuel Schneider, Bernhard Krauss and Martino Leghissa from Siemens Healthineers for support of measurements at the experimental low field system.
The project was funded by the German Federal Ministry of Education and Research under grant number 13GW0356B. The author is responsible for the content of this publication.
References
[1] Littin S, Gallichan D, Welz AM, et al. Monoplanar gradient system for imaging with nonlinear gradients. MAGMA. 2015;28(5):447-457. doi:10.1007/s10334-015-0481-8
[2] Jia F, Schultz G, Testud F, et al. Performance evaluation of matrix gradient coils. MAGMA. 2016;29(1):59-73. doi:10.1007/s10334-015-0519-y
[3] Layton KJ, Kroboth S, Jia F, et al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn Reson Med. 2017;77(4):1544-1552. doi:10.1002/mrm.26235
[4] https://pulseq.github.io/
[5] Haase A, Frahm J, Matthaei D, Hänicke W, Merboldt KD. FLASH imaging: rapid NMR imaging using low flip-angle pulses. 1986. J Magn Reson. 2011;213(2):533-541. doi:10.1016/j.jmr.2011.09.021
[6] Hennig, J., Nauerth, A. and Friedburg, H. (1986), RARE imaging: A fast imaging method for clinical MR. Magn Reson Med, 3: 823-833. https://doi.org/10.1002/mrm.1910030602
[7] Patz S, Wong ST, Roos MS. Missing pulse steady-state free precession. Magn Reson Med. 1989;10(2):194-209. doi:10.1002/mrm.1910100205
[8] Kobayashi N, Parkinson B, Idiyatullin D, et al. Development and
validation of 3D MP-SSFP to enable MRI in inhomogeneous magnetic fields.
Magn Reson Med. 2021;85(2):831-844. doi:10.1002/mrm.28469
Figures