1617
SPEN, RESOLVE and Spin-Echo EPI at 7T: A single-Tx human brain scan comparison1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Azrieli National Center for Brain Imaging, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Spatiotemporal encoding (SPEN) is an ultrafast imaging technique, that can serve as useful alternative to single shot and to read-out segmented (RESOLVE) spin-echo (SE) EPI methods, when trying to overcome image distortions due to magnetic field (B0) inhomogeneities. SPEN’s reliance on adiabatic sweeps could also make it an attractive candidate for imaging at high fields, and overcoming B1+-related inhomogeneities. Herein we present phantom and in-vivo results acquired on a 7T human imaging system for single and multi-shot SPEN acquisitions, corroborating its robustness vs comparable single and multi-shot SE-EPI and RESOLVE acquisitions implemented on a single-Tx configuration.
Introduction
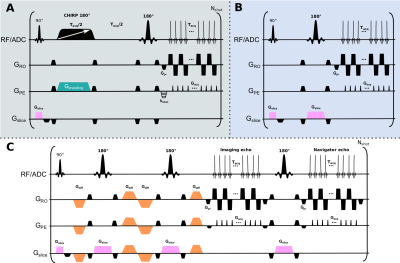
While increases in magnetic field results in sensitivity advantages, they are also associated with RF penetration problems,1 and on an aggravation of artifacts for sequences that, like EPI, are susceptible to off-resonance effects.2 The strong transmit B1 inhomogeneities that arise at 7T even for brain imaging,3 can be addressed by use of adiabatic pulses,4 dielectric pads,5 or parallel RF transmission6,7 (pTx) protocols. The latter is arguably the most flexible approach, but at the moment it lacks regulatory clearance for patient diagnostic imaging8 –limiting its use in commonly used techniques.Spatiotemporal encoding9 (SPEN) is an ultrafast imaging technique that has been used as alternative to both spin-echo (SE) EPI and to read-out segmented versions like RESOLVE, to overcome image distortions due to B0 inhomogeneities at 3T.10–12 In SPEN (Fig. 1A), spatial encoding is achieved by applying linearly frequency swept adiabatic pulses in conjunction with a gradient Gencoding, endowing spins excited by an initial slice-selective 90° pulse with a quadratic phase along the phase-encoding axis (y). This phase can be expressed as $$$\phi(y)=ay^{2}+by$$$, where a and b are constants under experimentalists control. Magnetization from the volume not pertaining to the imaged slice and hence not brought into transverse plane is also inverted by the swept pulse from +z to -z axis, but then promptly brought back to equilibrium by another non-selective 180° pulse, so as to avoid magnetization losses due to T1 relaxation when imaging multiple slices. SPEN’s use of two 180° pulses allows one to incorporate a twice-refocused diffusion encoding scheme,13 leading to a resemblance to multi-shot RESOLVE sequences (Fig. 1C). By contrast to EPI (Fig. 1B), where immunity to geometric distortions is defined by the bandwidth (BW) along phase-encoding dimension during decoding process and thus limited by minimal echo spacing (BWEPI=1/techo_spacing), SPEN‘s immunity to geometric distortions is defined by the encoding process; i.e., by the BW of the frequency swept pulse. SPEN’s use of adiabatic pulses for defining the BW also makes it an appealing candidate for ultrafast imaging at 7T, given these pulses’ ability to overcome the above mentioned B1+ RF inhomogeneity challenges. Based on these considerations, SPEN was compared against SE-EPI and RESOLVE variants on a 7T system using single-channel Tx irradiation modes.
Methods
Images were acquired at 7T on a Siemens Terra scanner using a single-channel transmit and 32-channel receiving head coil. The SPEN sequence and used in this study (Fig. 1A) followed the implementation presented in Ref. 15; SPEN data was processed as described in Ref. 10. The phantom used in this study is described in Ref. 16. Two human volunteers were scanned after signing suitable written consents.Results & Discussion
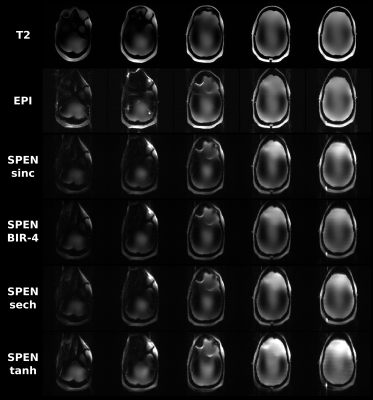
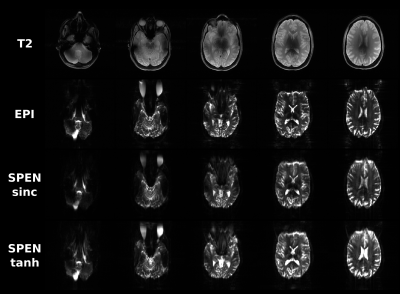
One of the main differences observed between the implementation of SPEN at 3T and 7T, was a signal intensity drop arising in the latter when using shorter TRs for multislice acquisitions. This led to cumulative effects affecting the signals after repeated application of the sequence: While no signal drop was observed for the first acquired slice, which always started from an equilibrium magnetization, consecutive slices showed progressively more pronounced effects as the order in slice acquisition increased. This endowed the non-selective 180˚ pulse with an importance that we did not see in 3T counterparts. This is illustrated in Figure 2, which compares T2 and SE-EPI images on a phantom, against an array of single-shot SPEN experiments collected with both the sinc inversion pulse used in original implementations, and adiabatic inversion pulses including BIR-4, sech and tanh; in general, the latter provided the best among the SPEN images.Figure 3 shows results obtained with such a variant and with a sinc pulse, on a healthy volunteer; also shown are T2 multiscan and SE-EPI single-shot counterparts. These SPEN and SE-EPI images show similar distortions and SNR –even if the former seems to have somewhat fewer artifacts when implemented with a tanh pulse.
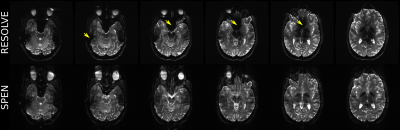
The advantages of SPEN’s reliance on adiabatic spatial encoding pulses become clearer upon comparing interleaved acquisitions using segmented acquisitions. SPEN does so along the PE direction, while the SE-EPI variant here used carried this out in a read-out segmented mode (RESOLVE; Fig. 1C). Figure 4 compares their resulting images. Notice that SPEN achieves a more uniform signal intensity over the whole brain, largely overcoming the spatial variations associated to B1+. On the other hand, as for the single-shot acquisitions, B0-associated distortions (e.g., in the posterior part of the brain for slices 5 and 6 in Fig. 4) are almost identical in SPEN and RESOLVE images –which were collected with nearly identical BWs.
Conclusion
SPEN shows potential as an alternative to EPI-based sequences at 7T, thanks to its robustness to B0 inhomogeneities, and to its ability to ameliorate B1+ penetration without relying on pTx. The latter arose from SPEN’s reliance on adiabatic sweeps, albeit at the cost of an increased SAR. Immunity to B0 variations was limited, and is a topic deserving further investigation.Acknowledgements
This work was funded by the Israel Science Foundation (grants 2508/17 and 965/18), the Minerva Foundation (Germany) and the generosity of the Perlman Family and the Azrieli Foundations.References
(1) Van de Moortele, P.-F.; Akgun, C.; Adriany, G.; Moeller, S.; Ritter, J.; Collins, C. M.; Smith, M. B.; Vaughan, J. T.; Uğurbil, K. Magn. Reson. Med. 2005, 54, 1503.
(2) Jezzard, P. Neuroimage 2012, 62, 648.
(3) Vaughan, J. T.; Garwood, M.; Collins, C. M.; Liu, W.; DelaBarre, L.; Adriany, G.; Andersen, P.; Merkle, H.; Goebel, R.; Smith, M. B.; Ugurbil, K. Magn. Reson. Med. 2001, 46, 24.
(4) Norris, D. G. Concepts Magn. Reson. 2002, 14, 89.
(5) Yang, Q. X.; Mao, W.; Wang, J.; Smith, M. B.; Lei, H.; Zhang, X.; Ugurbil, K.; Chen, W. J. Magn. Reson. Imaging 2006, 24, 197.
(6) Hoult, D. I.; Phil, D. J. Magn. Reson. Imaging 2000, 12, 46.
(7) Ibrahim, T. S.; Lee, R.; Baertlein, B. A.; Kangarlu, A.; Robitaille, P. L. Magn. Reson. Imaging 2000, 18, 733.
(8) Fagan, A. J.; Bitz, A. K.; Björkman-Burtscher, I. M.; Collins, C. M.; Kimbrell, V.; Raaijmakers, A. J. E.; ISMRM Safety Committee. J. Magn. Reson. Imaging 2021, 53, 333.
(9) Shrot, Y.; Frydman, L. J. Magn. Reson. 2005, 172, 179.
(10) Cousin, S. F.; Liberman, G.; Solomon, E.; Otikovs, M.; Frydman, L. Magn. Reson. Med. 2019, 82, 1322.
(11) Otikovs, M.; Nissan, N.; Furman-Haran, E.; Anaby, D.; Allweis, T. M.; Agassi, R.; Sklair-Levy, M.; Frydman, L. J. Magn. Reson. Imaging 2021, 53, 1913.
(12) Solomon, E.; Liberman, G.; Nissan, N.; Furman-Haran, E.; Sklair-Levy, M.; Frydman, L. Magn. Reson. Med. 2020, 84, 1391.
(13) Reese, T. G.; Heid, O.; Weisskoff, R. M.; Wedeen, V. J. Magn. Reson. Med. 2003, 49, 177.
(14) Schmidt, R.; Frydman, L. Magn. Reson. Med. 2014, 71, 711.
(15) Liberman, G.; Solomon, E.; Lustig, M.; Frydman, L. Magn. Reson. Med. 2018, 79, 796.
(16) Jona, G.; Furman-Haran, E.; Schmidt, R. NMR Biomed. 2021, 34, e4421.
Figures