1602
MRI Relaxation Times Change in the Metaphysis Following Ischemic Injury to the Femoral Head: An In Vivo Piglet Model Study1Veterinary Clinical Sciences, University of Minnesota, ST. Paul, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Quantitative mapping of T2, T1ρ, adiabatic T1ρ, and adiabatic T2ρ relaxation times may be useful to assess ischemic injury to the femoral head. In this work, we investigated whether these relaxation times are sensitive in detecting compositional changes to the primary spongiosa (the region of the metaphysis adjacent to the growth plate) following ischemic injury to the femoral head in a piglet model. We found that T1ρ and adiabatic T2ρ decreased in the primary spongiosa following ischemic injury to the femoral epiphysis, which suggests these methods may be useful to assess femoral head growth disturbances following ischemic injury.
Introduction
Quantitative mappings of T2, T1ρ, adiabatic T1ρ (aT1ρ), and adiabatic T2ρ (aT2ρ) relaxation times have been shown to be sensitive in detecting early-stage ischemic injury to the femoral head in ex vivo and in vivo studies of a piglet model of Legg-Calvé-Perthes disease (LCPD) 1-4, a pediatric hip disorder involving disruption of blood supply to the femoral head that can lead to joint deformation and osteoarthritis 5. In particular, the relaxation times increase in the secondary ossification center (i.e., bone and bone marrow of the femoral epiphysis, proximal to the growth plate) as early as 48 hours after surgical induction of global femoral head ischemia 1-4. It has previously been assumed that the metaphysis (i.e., bone and bone marrow distal to the growth plate) is unaffected by the surgery, and relaxation times were unaltered in a large metaphyseal region of interest following ischemic injury to the epiphysis 1-4. However, a subregion of the metaphysis called the primary spongiosa (PS), which is adjacent to the growth plate and is a metabolically active vascularized region composed of cartilage and woven bone 6, warrants closer investigation. The PS has been shown in histological analyses to be thinned following growth arrest in the piglet model by one week after onset of ischemia 7. Concomitantly, T1ρ relaxation times are particularly long in the PS 3 which may indicate their sensitivity to compositional changes in this region. The purpose of this study was to determine whether T2, T1ρ, aT1ρ, and aT2ρ change in the PS following ischemic injury to the femoral epiphysis in the piglet model one week post-operatively.Methods
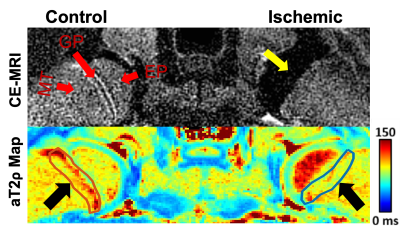
Animal Model: This study was approved by our institution’s IACUC. Ten piglets underwent surgery at six weeks of age to completely interrupt the vascular supply to one of the femoral heads by placement of a ligature around the femoral neck and transection of the ligamentum teres 8. The contralateral femoral head was unoperated and served as a control.In Vivo 3T MRI: One week after surgery, both femoral heads of each piglet were imaged in vivo at 3T MRI using: (i) T2, T1ρ, aT1ρ, and aT2ρ mapping with a magnetization-prepared 2D TSE sequence (T2: MLEV-4 preparation; T1ρ: 350 Hz spin-lock preparation; aT1ρ and aT2ρ: 1000 Hz, 6.0 ms HS1 pulse train preparation; T2 and T1ρ prep times = 0/20/40/60/80 ms; and aT1ρ and aT2ρ prep times = 0/24/48/72/96 ms); and (ii) subtraction contrast-enhanced MRI (CE-MRI) following intravenous injection of 0.2 mmol/kg gadoteridol to confirm lack of perfusion in the operated femoral heads.
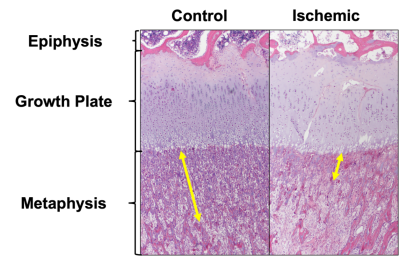
Histology: Two of the 10 piglets were euthanized immediately following the MRI exam, and the femoral heads were harvested for histological analysis. A board-certified veterinary pathologist performed histological analyses of H&E-stained sections of the femoral heads.
Data Analysis: A region of interest (ROI) constituting the metaphysis immediately adjacent to the physis (primary spongiosa [PS]) was manually drawn using T2-weighted images. Median T2, T1ρ, aT1ρ, and aT2ρ relaxation times were measured in each ROI, and their percent change in the ischemic vs. control femoral heads was calculated and statistically analyzed using paired t-tests with a significance threshold of p<0.0125. The Cohen’s d effect sizes of the methods were also compared to assess their relative reliabilities.
Results
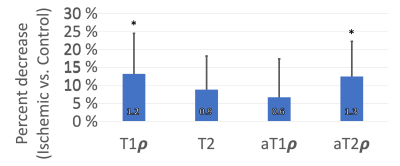
Contrast-enhanced MRI confirmed the absence of femoral head perfusion in the operated limb in each piglet. T1ρ and aT2ρ relaxation times were significantly decreased in the PS of the ischemic vs. control femoral heads (Figure 1). T1ρ and aT2ρ decreased on average 13.2±11.3% (p=0.0065; d=1.2) and 12.5±9.8% (p = 0.0032; d=1.3, Figure 2). T2 and aT1ρ were on average decreased but did not reach statistical significance: 8.8±9.3% (p = 0.0132; d=0.9) and 6.7±10.7% (p=0.0926; d=0.6. Histological analysis identified evidence of thinning of the PS in the ischemic vs. control femoral heads (Figure 3).Discussion
Our findings demonstrate that T1ρ and aT2ρ mapping are sensitive to changes to the primary spongiosa following ischemic injury to the femoral head. Based on histological results, the relaxation times may decrease on account of altered conversion of the PS to secondary spongiosa (which has more mature, mineralized bone, is less vascularized, and has reduced cellularity) due to disruption of growth plate function. It has previously been conjectured that T1ρ may be particularly useful to assess changes to the long relaxation time components of the PS (such as blood vessels and marrow cells) due to reduced sensitivity to mineralization relative to T2 3. Our findings further support that T1ρ (and aT2ρ) may provide unique sensitivity to identify alterations in the endochondral ossification adjacent to the growth plates, and thus may help inform the severity of injury and disruption to bone growth in ischemic joint disorders such as LCPD.Conclusion
T1ρ and aT2ρ relaxation time mapping are sensitive in detecting changes to the primary spongiosa that may inform the extent of injury and growth disturbance to the developing femoral head.Acknowledgements
This project was supported by NIH grants R56AR078315, K01AR070894, UL1TR002494, K01OD021293, T32OD010993, and P41EB027061. We thank Kathy Stuebner, Amber Winter, Kelly Bergsrud, Andrea Chehadeh, Sara Pracht, Katalin Kovacs, Paula Overn, and Dee Koski for their assistance.References
1. Johnson CP, Wang L, Tóth F, Aruwajoye O, Carlson CS, Kim HK, Ellermann JM. Quantitative MRI helps to detect hip ischemia: preclinical model of Legg- Calvé-Perthes disease. Radiology 2018; 289(2):386-395.
2. Johnson CP, Tóth F, Carlson CS, Armstrong AR, Zbýň Š, Wu B, Ellermann JM, Kim HK. T1ρ and T2 mapping detect acute ischemic injury in a piglet model of Legg–Calvé–Perthes disease. J Orthop Res 2021 (epub).
3. Johnson CP, Toth F, Armstrong AR, Kim HK, Ellermann JM. Quantitative T2, T1ρ, and diffusion mapping of early-stage ischemic osteonecrosis of the femoral head: an in vivo piglet model study at 3T MRI. Proc 28th ISMRM Annual Meeting 2020; No. 1145.
4. Johnson CP, Bhave S, Armstrong AR, Tóth F. Utility of adiabatic T1ρ and T2ρ mapping to detect ischemic injury to the femoral head: an in vivo piglet model study at 3T MRI. Proc 29th ISMRM Annual Meeting 2021; No. 0154.
5. Kim HK. Pathophysiology and new strategies for the treatment of Legg-Calve-Perthes disease. J Bone Joint Surg Am 2012; 94:659-669.
6. Mackie E, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The international journal of biochemistry & cell biology. 2008 Jan 1;40(1):46-62.
7. Armstrong AR, Tóth F, Carlson CS, Kim HK, Johnson CP. Early changes to the growth plate zones and primary spongiosa secondary to femoral head ischemia in a piglet model of Legg-Calvé-Perthes disease. Proc ORS Annual Meeting 2022 (submitted).
8. Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84-A(8):1329-34.
Figures