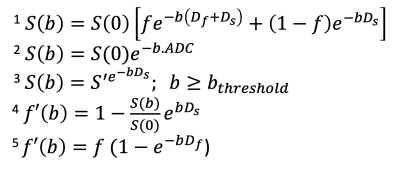1598
IVIM Detects Bone Ischemia in a Piglet Model of Legg-Calvé-Perthes Disease1Veterinary Clinical Sciences, University of Minnesota, St. Paul, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Intravoxel incoherent motion (IVIM) is a promising method to measure both tissue diffusion and perfusion using a single multi b-value diffusion-weighted imaging (DWI) acquisition. In this work, we investigated whether IVIM is sensitive in detecting surgically-induced femoral head ischemia in a piglet model of avascular necrosis. We found that the IVIM perfusion coefficient (Df) and fraction (f) decreased in the ischemic vs. contralateral-control femoral heads. Conversely, the IVIM diffusion coefficient (Ds) increased as a result of subsequent injury to the operated femoral head. These findings suggest that IVIM may provide a non-contrast-enhanced means to assess bone ischemia and perfusion.
Introduction
Legg-Calvé-Perthes disease (LCPD) is a pediatric hip disorder caused by vascular interruption to the developing femoral head, which can lead to bone necrosis, joint deformation, and osteoarthritis1. Detection of LCPD in the early avascular stage relies on gadolinium-based contrast-enhanced MRI (CE-MRI) to confirm femoral head ischemia and measure the degree of femoral head involvement2. However, there is interest in alternative, non-contrast-enhanced approaches to measure femoral head perfusion due in part to concerns about potential long-term effects of gadolinium in pediatric patients2,3. One potential alternative approach is intravoxel incoherent motion (IVIM), originally proposed by Le Bihan et al.4, which uses a two-compartment model (Equation 1) to separate and simultaneously measure tissue diffusion coefficient (Ds), perfusion coefficient (Df), and perfusion fraction (f) using a multi b-value diffusion-weighted imaging (DWI) acquisition. IVIM has been utilized in multiple organs and tissues, primarily those that are highly perfused5,6. However, the femoral head (and bone and bone marrow in general) presents a challenge for IVIM in that baseline perfusion is low7. Furthermore, prior attempts to measure IVIM in the bone have not had a means to validate observed effects8. The purpose of our study was to determine whether IVIM can detect femoral head ischemia in a piglet model of LCPD. The piglet model involves surgical induction of global femoral head ischemia, while the unoperated contralateral femoral head is a perfused control. The piglet model thus provides a controlled experiment to determine whether a reduction in femoral head perfusion is detectable using IVIM. We hypothesized that IVIM-measured perfusion (f*Df) would be decreased in the operated vs. control femoral heads one week after surgery.Methods
Animal Model: Our institution’s IACUC approved this study. Ten piglets, age six weeks old, underwent surgery to interrupt the blood supply globally to one femoral head by placing a ligature around the femoral neck and transecting the ligamentum teres9 No surgery was performed on the contralateral femoral head, which served as a control.In Vivo 3T MRI: The bilateral hips of the piglets were imaged in vivo at 3T MRI one week following surgery, using: (i) RESOLVE DWI with 13 b-values (0,20,30,40,50,60,70,80,90,100,200,300,500 s/mm2), TR/TE1/TE2 = 2500/68/122 ms, EPI factor = 94, spatial resolution = 1.1x1.1 mm2, slice thickness = 2.0 mm, and fat sat; and (ii) gadolinium-based contrast-enhanced MRI to confirm surgical induction of ischemia (lack of femoral head perfusion) in the operated femoral heads.
Data Analysis: Regions of interest (ROIs) constituting the secondary ossification centers (SOCs; i.e., the bone and bone marrow) of the operated and control femoral heads were manually drawn using the b=0 diffusion-weighted image at a single central slice. The median ROI values for each of the 13 b-value images for a given femoral head were measured and used as the IVIM signals (S(b)). ADC was then estimated from IVIM signals using a mono-exponential function (Equation 2). The analytical segmented (AS) method10,11 was then used to extract IVIM parameters due to its higher performance in low perfusion regimes than alternative techniques10. In AS, first, Ds is obtained by mono-exponentially fitting the IVIM signals with b ≥ bthreshold (in our case, 100 s/mm2) according to Equation 3. Second, f’(b) is determined using Equation 4, and then f and Df are extracted using Equation 5. ADC and IVIM parameter (Ds, Df, and f) values were then calculated and statistically compared between the ischemic vs. control femoral heads across the ten piglets using paired t-tests (p<0.05). Their percent changes and effect sizes (Cohen’s d) were also assessed.
Results
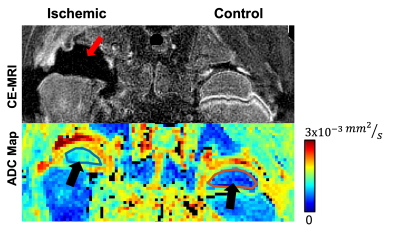
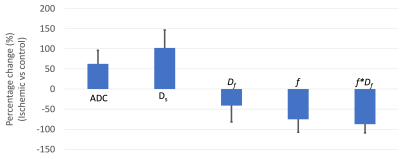
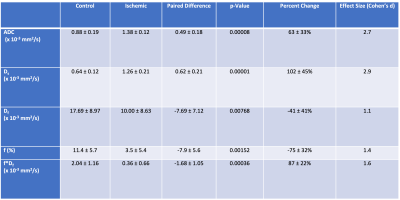
Lack of perfusion in the operated femoral head was confirmed by contrast-enhanced MRI in all ten piglets (Figure 1). IVIM diffusion parameters (ADC and Ds) were significantly increased in the SOC of the ischemic vs. control femoral heads (Figures 1 and 2 and Table 1), increasing on average 64±33% (p=0.00008; d=2.7) and 102±45% (p = 0.00001; d=2.9), respectively. In contrast, IVIM perfusion parameters (Df, f, and f*Df) decreased significantly in the operated vs. control femoral heads (Table 1 and Figure 2). On average, Df, f, and f*Df decreased by -41±41% (p = 0.00768; d=1.1), -75±32% (p=0.00152; d=1.4) and -87±22% (p=0.00036; d=1.6), respectively. The parameter f*Df decreased in all 10 operated femoral heads vs. their contralateral controls.Discussion
Our findings demonstrate that IVIM parameters (Ds, Df, f, and f*Df) are sensitive in detecting ischemia and subsequent injury to the femoral head. IVIM perfusion parameters Df, f, and f*Df consistently decreased in the ischemic vs. control femoral heads, demonstrating that IVIM may provide a non-contrast-enhanced means to assess ischemia and perfusion changes in bone. The IVIM blood flow metric f*Df had a greater effect size than f or Df alone. Furthermore, Ds was more sensitive than ADC to ischemic injury on account of Ds ignoring low b-values, which have perfusion components that can overestimate ADC in the control femoral heads.Conclusion
IVIM is sensitive to ischemia and subsequent injury to the femoral head. In particular, the IVIM perfusion parameter f*Df has potential to be used as a non-contrast-enhanced approach to assess bone perfusion.Acknowledgements
This project was supported by NIH grants R56AR078315, K01AR070894, UL1TR002494, K01OD021293, and P41EB027061. We thank Kathy Stuebner, Amber Winter, Kelly Bergsrud, Andrea Chehadeh, Sara Pracht, and Dee Koski for their assistance.References
1. Kim HK. Pathophysiology and new strategies for the treatment of Legg-Calve-Perthes disease. J Bone Joint Surg Am 2012; 94:659-669.
2. Laine JC, Martin BD, Novotny SA, Kelly DM. Role of Advanced Imaging in the Diagnosis and Management of Active Legg-Calvé-Perthes Disease. J Am Acad Orthop Surg. 2018 Aug 1;26(15):526-536.
3. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017 Jul;16(7):564-570.
4. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505.
5. Barbieri S, Donati OF, Froehlich JM, Thoeny HC. Impact of the calculation algorithm on biexponential fitting of diffusion‐weighted MRI in upper abdominal organs. Magnetic resonance in medicine. 2016 May;75(5):2175-84.
6. Federau C. Measuring Perfusion: Intravoxel Incoherent Motion MR Imaging. Magn Reson Imaging Clin N Am. 2021 May;29(2):233-242. doi: 10.1016/j.mric.2021.01.003. PMID: 33902905.
7. Meeus EM, Novak J, Withey SB, Zarinabad N, Dehghani H, Peet AC. Evaluation of intravoxel incoherent motion fitting methods in low‐perfused tissue. Journal of magnetic resonance imaging. 2017 May;45(5):1325-34.
8. Meng XH, Zhang XN, Wang Z, Yang JP, Zhang ZL, Xia S. Increased diffusion and decreased perfusion in epiphyses of femoral heads detected via intravoxel incoherent motion after closed reduction in children with developmental dysplasia of the hip. Acta Radiologica. 2018 Sep;59(9):1130-8.
9. Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84-A(8):1329-34.
10. Erick Buko, J. Zhang, A. Ajala, PH. Hor, and R. Muthupillai. An analytical segmented (AS) approach for extracting intravoxel incoherent motion (IVIM) model parameters. Proc. 26th ISMRM Annual Meeting 2018; No. 5361.
11. Erick O Buko , Afis Ajala , Jiming Zhang , Pei Herng Hor , and Raja Muthupillai. An analytical segmented approach for extracting TE independent perfusion fraction in intravoxel incoherent motion (IVIM) MRI Proc. 28th ISMRM Annual Meeting 2020; No. 6625.
Figures