1594
How much earlier did MRI detect breast cancer than mammography in the DENSE trial?1., Amsterdam, Netherlands
Synopsis
The current analysis found MRI screening in the DENSE trial detected invasive breast cancers about 6 years earlier than mammography. The calculation is based on first and second round invasive cancer detection rates. The 6 years is also consistent with the trial’s high false positive rate in the second round that used “prior” first round exams to estimate lesion growth over the 2 years between rounds. The “how much earlier” measure provides an additional means for comparing the relative performance of breast cancer screening modalities.
INTRODUCTION
The DENSE trial [BakkerMF2019, VeenhuizenSGA2021] is the most extensive clinical comparison of MRI screening for breast cancer with mammography. The trial cohort was comprised of only women with extremely dense breasts. This condition applies to 8% of the female population of the Netherlands – where the trial was conducted.The DENSE trial found MRI screening detected breast cancers 14 times smaller in volume than mammography—a reduction in median cancer size from 17mm to 7mm. The trial also found the interval cancer rate reduced from 5.0 per 1000 for mammography to 0.8 per 1000 for MRI screening. Both these exceptional results strongly suggest a much better outcome for patients who have their cancers detected with MRI screening. However, 5 to 10 years of follow-up is needed to confirm this promising outcome. The high false positive detection rate of MRI screening is the main reason the much earlier detection of breast cancer with MRI screening is not widely available [ACS2021, VerburgE2020, DekkerBM2021, CoverKS2021].
In addition to cancer size and interval cancer rate, a third measure of the relative performance of MRI screening and mammography is how much earlier breast cancers are detected by MRI than mammography. “How much earlier”, in years, maybe a useful third measure against which to correlate patient outcomes to modalities. It also provides a more intuitive representation of the superior sensitivity of MRI and MRI’s promise of improved patient outcome.
METHODS
All of the numerical values used in the current analysis are taken from the first and second round publications of the DENSE trial [BakkerMF2019, VeenhuizenSGA2021].The first round of the DENSE trial screened 32,312 women with extremely dense breasts using mammography. An additional 4,783 women, also with extremely dense breasts, were screened with MRI within a few months of receiving a negative mammography examination. The median size of the cancers detected with mammography was 17mm – which does not include the larger interval cancers [BakkerMF2019]. The cancer detection rate was 13.1 per 1000 for invasive cancers [VeenhuizenSGA2021].
In the second round of the DENSE trial, 3,436 women completed an MRI exam after having completed the first round successfully. Each MRI exam in the second round was read in combination with the “prior” MRI exam from the first round to allow the growth of each lesion over the 2 years between rounds to be estimated. The cancer detection rate of the second round was 4.1 per 1000 for invasive cancers. The median size of the cancers detected in the second round was 7mm [VeenhuizenSGA2021]. The false positive detection rate for invasive cancers was 28.1 per 1000 or about 7 false positives for each invasive cancer detected.
All MRI exams were acquired within 3 months of a negative result on mammography.
RESULTS
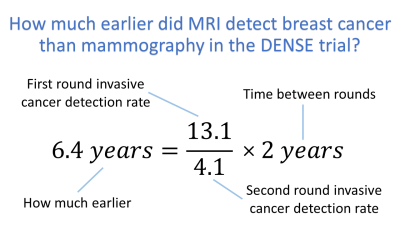
Figure 1 shows the equation and the value of the “how much earlier” estimate of 6.4 years. While a simple equation, it is based on key understandings and simplifications.One understanding is the second round invasive cancer detection rate, to a good approximation, is the number of cancers that occurred every two years. While the timing of cancer detection depends on the screening modality, the rate of occurrence is assumed to be constant over time.
A second understanding is the first round invasive cancer detection rate is the number of cancers missed by mammography but detected by MRI. If the mammogram 3 months prior to each MRI had actually been an MRI, the rate would have been zero to within measurement error.
A key simplification is assuming MRI’s superior sensitivity is only because MRI can see smaller cancers than mammography and mammography misses cancers only because they are too small for it.
DISCUSSION
The high false positive detection rate of the second round allows a lower bound to be placed on how much earlier MRI detected breast cancer than mammography in the DENSE trial. The second round used MRI exams from the first round to determine if there was any significant growth in each lesion over the 2 years from the first to the second round. If MRI actually detected breast cancers only 2 years earlier than mammography, cancers would have typically grown from the 7mm median size of MRI detection to the 17mm median size of mammography over the 2 years. A benign lesion would have remained at 7mm in size. Therefore, radiologists would have little difficulty discriminating malignant from benign lesions and the false positive rate would have been much lower. Consequently, MRI must detect cancers much more than 2 years earlier than mammography in the DENSE trial. This lower bound is consistent with the 6 years calculated from the cancer detection rates.The approximations made - such as no cancers detected by mammography or as interval cancers during the study - while not strictly correct, were reasonable. The differing sensitivity of MRI and mammography to different cancer types may need further examination.
CONCLUSION
At about 6 years, the earlier detection by MRI of breast cancer than mammography provides another measure to compare the performance of the two breast cancer screening modalities. In addition, it may be possible to use the “how much earlier” measure to compare other modalities.Acknowledgements
No acknowledgement found.References
ACS2021. American Cancer Society. Breast MRI for Screening. Accessed on November 11, 2021. Available from: https://www.breastcancer.org/symptoms/testing/types/mri/screening
BakkerMF2019. Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, Emaus MJ, Loo CE, Bisschops RHC, Lobbes MBI, de Jong MDF, Duvivier KM, Veltman J, Karssemeijer N, de Koning HJ, van Diest PJ, Mali WPTM, van den Bosch MAAJ, Veldhuis WB, van Gils CH; DENSE Trial Study Group. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N Engl J Med. 2019;381:2091-2102.
CoverKS2021. Cover KS. Is It Possible for MRI Screening of Breast Cancer to be Available to Many More Women by Greatly Reducing its False Positive Detections via Ultrafast Time to Enhancement Measurements? J Magn Reson Imaging. 2021 Oct 22. doi: 10.1002/jmri.27969. Online ahead of print
DekkerBM2021. den Dekker BM, Bakker MF, de Lange SV, Veldhuis WB, van Diest PJ, Duvivier KM, Lobbes MBI, Loo CE, Mann RM, Monninkhof EM, Veltman J, Pijnappel RM, van Gils CH; Reducing False-Positive Screening MRI Rate in Women with Extremely Dense Breasts Using Prediction Models Based on Data from the DENSE Trial. Radiology. 2021;301:283-29
VeenhuizenSGA2021. Veenhuizen SGA, de Lange SV, Bakker MF, Pijnappel RM, Mann RM, Monninkhof EM, Emaus MJ, de Koekkoek-Doll PK, Bisschops RHC, Lobbes MBI, de Jong MDF, Duvivier KM, Veltman J, Karssemeijer N, de Koning HJ, van Diest PJ, Mali WPTM, van den Bosch MAAJ, van Gils CH, Veldhuis WB; DENSE Trial Study Group. Supplemental Breast MRI for Women with Extremely Dense Breasts: Results of the Second Screening Round of the DENSE Trial. Radiology. 2021;299:278-286.
VerburgE2020. Verburg E, van Gils CH, Bakker MF, Viergever MA, Pijnappel RM, Veldhuis WB, Gilhuijs KGA. Computer-Aided Diagnosis in Multiparametric Magnetic Resonance Imaging Screening of Women With Extremely Dense Breasts to Reduce False-Positive Diagnoses. Invest Radiol. 2020;55:438-444.