1591
Magnetic Resonance Elastography for Early Assessment of Response to Neoadjuvant Chemotherapy in Women with Breast Cancer1School of Cancer & Pharmaceutical Sciences, King's College London, London, United Kingdom, 2Oncoplastic breast surgery, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom, 3School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 4Inserm U1148, LVTS, University Paris Diderot, Paris, France, 5MR Research Collaborations, Siemens Healthcare Ltd., Frimley, United Kingdom, 6Oncology and Haematology Clinical Trials, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom, 7MRI department, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom, 8Breast Radiology, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom, 9Breast Radiology, King's College Hospital NHS Foundation, London, United Kingdom, 10Histopathology, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom, 11Medical Oncology, Guy's & St. Thomas' NHS Foundation Trust, London, United Kingdom
Synopsis
Approximately one-third of women with breast cancer undergoing neoadjuvant chemotherapy (NACT) have no discernible disease response to their treatment yet are subjected to the potential side-effects of their treatment. Currently, response to NACT is assessed by determining tumour size, contrast avidity, and lymph node involvement on MRI pre-, mid- and post-treatment. As a result, women undergo 3-4 cycles of NACT with unknown efficacy. Changes in a tumour’s biomechanics as assessed by magnetic resonance elastography (MRE) after only one cycle of NACT correlate with response to treatment, allowing for early assessment of a patient’s response to NACT.
Introduction
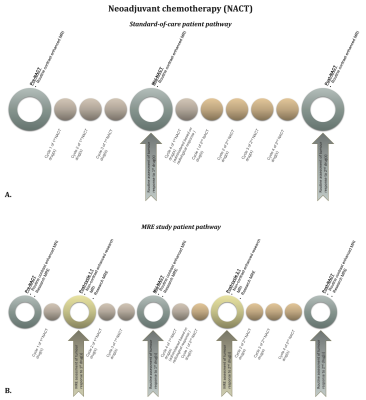
Women with locally advanced, metastatic, triple negative or inoperable breast cancer, including young women with lymph node positive disease, are amenable to chemotherapy prior to surgery - neoadjuvant chemotherapy (NACT) - with the aim to downstage their cancer1–4. Typically, response to NACT is determined by assessing the tumour size, lymph node involvement and avidity of contrast enhancement on magnetic resonance imaging (MRI) at baseline (pre-NACT), after 3 cycles (mid-NACT) and at the end of chemotherapy (post-NACT) (Figure 1A). This requires a discernible change in size or uptake of a contrast agent to occur2–4. As a result, women would have received 3-4 cycles of each chemotherapy regimen before the efficacy can be determined. Ultimately, 31% of the breast cancer patients undergoing NACT will have no beneficial response or even disease progression3. Therefore, early identification of treatment resistance patients is pivotal in tailoring treatment to individual patients, thereby minimizing unnecessary toxicity and facilitating a better therapeutic response.By fully integrating magnetic resonance elastography (MRE) into a routine MRI scanner, the tumour’s biomechanical properties can be assessed as early indicators of therapy response8. MRE is performed in addition to standard T1/T2-weighted, diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) anatomical sequences9–12.
Methods
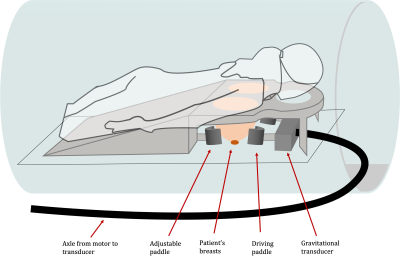
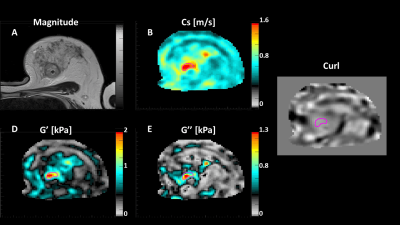
Women ≥18 years, diagnosed with invasive breast cancer and scheduled to undergo NACT were recruited. MRE was carried out at 5 timepoints, 3 as part of the patients’ standard-of-care MRI scans and 2 additional MRE scans were performed post-cycle 1 of the first drug regimen and, following changing to a new NACT regimen, post-cycle 1 of the second drug(s) (Figure 1B). A routine breast biopsy RF-coil was used as the open design allowed for the addition of the MRE transducer, which produced mechanical waves in the breasts by driving two adjustable paddles in cranio-caudal direction13 (Figure 2). Clinical images were obtained on a 1.5T System Area (Siemens Healthcare, Germany) using the standard-of-care MR sequences. Guided by the anatomical MR images, MRE sequences were centred on the tumour core. The MRE data was acquired using the eXpresso13 sequence with 36 Hz actuation frequency14, 7 wave-phase offsets and fractional motion-encoding gradients15 at 20 mT/m strength. Imaging parameters of the protocol were: 16 slices, 3mm isotropic voxel size, a 128 x 128 acquisition matrix, GRAPPA acceleration factor of 2, resulting in a FOV of 384 x 384 x 48 mm3, and TE = 9.2ms in-phase. The total duration of the MRE scan was 7:17 minutes.MRE informed about the biomechanics of the tumour, providing e.g., wave speed (cs [m/s]), storage modulus (G' [kPa]), loss modulus (G'' [kPa]) and phase angle ((2/π) * atan(G''/G')). Viscoelastic parameters were reconstructed from the displacement field by local inversion of the complex wave equation. A Gaussian filter was applied prior to the inversion to insure smoother spatial derivatives which were subsequently calculated in Fourier space16 (Figure 3).
Changes in biomechanical properties – in particular the wave speed cs – of the tumour between the pre-NACT and post-cycle 1.1 MRE scans and between mid-NACT and post-cycle 2.1 MRE scans were used to determine the treatment response. MRE findings were correlated with response to NACT, as determined by standard-of-care MRI assessment mid- and post-NACT.
Results
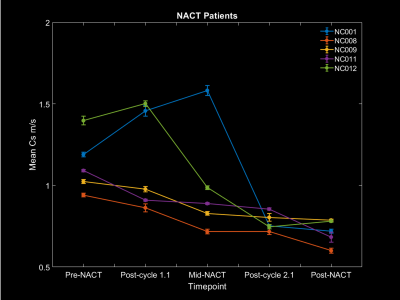
Forty women were recruited between September 2020 and October 2021. Of those, five women have now completed their NACT and their corresponding imaging (n=25 scans). Three women (NC008, NC009 and NC011) had a partial or complete response to their first NACT regimen. Their MRE derived mean wave speed in the tumour decreased following one cycle of NACT. Two women (NC001 and NC012) had disease progression whilst undergoing their first NACT regimen. The tumour’s mean wave speed increased between their pre-NACT and post-cycle 1.1 MRE scan. All women had a partial or complete response to their second NACT regimen, corresponding with a diminution in wave speed from mid-NACT to post-cycle 2.1 (Figure 4).Discussion
This preliminary interim analysis was performed on a relatively small dataset and will have to be repeated once the full dataset, once completed. However, the mean wave speed results from these 25 scans were significant within 1 sigma. Since for this analysis only the wave speed has been explored, comparisons between treatment response and early changes in the other biomechanical tumour properties will need to be investigated.Conclusion
Early analysis of 25 MRE scans indicates that variations in the wave speed within a tumour as assessed by MRE after the first cycle of a NACT regimen, correlates with the treatment response as determined by standard-of-care MR imaging. Therefore, changes in MRE derived wave speed could be an early indication of a patient’s response to NACT.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement 668039.
References
1. Asaoka, M., Gandhi, S., Ishikawa, T. & Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer Basic Clin. Res. 14, (2020).
2. Wang, H. & Mao, X. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des. Devel. Ther. 14, 2423–2433 (2020).
3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. The Lancet.Oncology 19, 27–39 (2018).
4. Fowler, A. M., Mankoff, D. A. & Joe, B. N. Imaging Neoadjuvant Therapy Response in Breast Cancer. Radiology 285, 358–375 (2017).
5. Heldin, C. H., Rubin, K., Pietras, K. & Östman, A. High interstitial fluid pressure - An obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004).
6. Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006).
7. Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
8. Fovargue, D. et al. Towards noninvasive estimation of tumour pressure by utilising MR elastography and nonlinear biomechanical models: a simulation and phantom study. Sci. Rep. 10, 1–13 (2020).
9. Muthupillai, R. et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (80-. ). 269, 1854–1857 (1995).
10. Sinkus, R. et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 23, 159–65 (2005).
11. Sinkus, R. et al. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn. Reson. Med. 58, 1135–1144 (2007).
12. Xu, L. et al. Magnetic resonance elastography of brain tumors: preliminary results. Acta radiol. 48, 327–330 (2007).
13. Garteiser, P. et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed. 26, 1326–1335 (2013).
14. Runge, J. H. et al. A novel magnetic resonance elastography transducer concept based on a rotational eccentric mass: Preliminary experiences with the gravitational transducer. Phys. Med. Biol. 64, (2019).
15. Rump, J., Klatt, D., Braun, J., Warmuth, C. & Sack, I. Fractional encoding of harmonic motions in MR elastography. Magn. Reson. Med. 57, 388–395 (2007).
16. Sinkus, R. et al. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn. Reson. Med. 53, 372–387 (2005).
Figures