1582
Arterial Input Function Estimation Using All-Channel Blind Deconvolution with Spatial Regularization in DCE-MRI1Institute of Scientific Instruments, Czech Academy of Sciences, Brno, Czech Republic, 2Institute of Information Theory and Automation, Czech Academy of Sciences, Praha, Czech Republic
Synopsis
This work is focused on estimation of an arterial input function (AIF) in DCE-MRI. We propose a new concept of blind deconvolution to use as much available information as possible. In contrast to multi-channel blind deconvolution based on processing of selected tissue signals, our all-channel blind deconvolution uses tissue signals of all voxels within the studied tissue to simultaneously estimate the AIF and perfusion maps. In addition, edge-preserving spatial regularization is included in the perfusion-map estimation scheme. The method is illustrated on DCE-MRI recordings of tumor-bearing mice.
INTRODUCTION
In quantitative DCE-MRI, reliable estimation of the arterial input function (AIF) is one of crucial tasks, which is still unsolved, especially in small-animal applications. Blind deconvolution1 is an approach potentially solving the problems of AIFs measured in large arteries (flow, partial-volume, saturation, dispersion artifacts) or population-based AIFs (ignoring subject-specific AIF variations). However, it is often an ill-conditioned or even ill-posed inverse problem, which calls for use of some additional information.In multi-channel blind deconvolution, several representative regions of interest (ROIs-channels) and their tissue curves are selected manually2, randomly3 or using clustering1 and fitted with a pharmacokinetic model, estimating both terms of its convolutional form: the AIF (assumed to be the same for all channels) and the residual functions (parametrized by perfusion parameters). The estimated AIF is then used in standard voxel-by-voxel non-blind deconvolution to estimate perfusion-parameter maps. It is known that for different choices of the ROIs for blind deconvolution, different AIF may be obtained3.
METHODS
We propose “all-channel” blind deconvolution where all voxel-based tissue curves within the analyzed tissue (e.g. the whole tumor) are used as separate channels. This leads to simultaneous estimation of perfusion-parameter maps and the AIF. It is formulated as iterative optimization, alternating between updates of the perfusion maps and updates of the AIF. In addition, edge-preserving similarity of neighboring voxels in perfusion maps is induced by spatial regularization4.The proposed methodology is evaluated on 2x3 tumor-mice recordings: 3 mice (SHO mice with MiaPaCa-2 luc SPX2 ovarian tumor cells, subcutaneously implanted in the right flank), each with 2 DCE recordings – one with a low-molecular-weight contrast agent (Magnevist, Bayer HealthCare Pharmaceuticals, Germany) and one with a high-molecular-weight contrast agent (GadoSpin-P, Miltenyi Biotec, Germany), approved by the National Animal-Research Authority, measured with a 9.4T Bruker BioSpin (Bruker Biospin MRI, Germany) scanner using a 2D golden-angle spoiled gradient echo sequence (TR/TE=15/1.5ms, FA=20°). Image reconstruction was done using nonuniform fast Fourier transform, temporal resolution 0.9s. For perfusion analysis, a 7-parameter AIF model5 and the TH6,7 residual-function model were used.
The regularized all-channel blind deconvolution method was compared with the same method with no spatial regularization and with multi-channel blind deconvolution where the channels were determined using k-means clustering1 (initially 12 clusters, subsequently several clusters were discarded due to corrupted signals, resulting in 6 to 11 channels per recording).
RESULTS
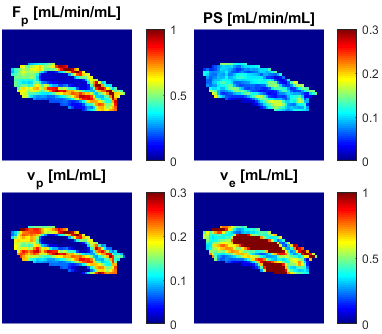
All tested methods showed consistent differences between the Magnevist and GadoSpin-P AIFs (Fig.1) – a slower decay of GadoSpin-P AIF tail due to its slower renal clearance. The level of the AIF peak is expected to be independent of the contrast agent, which corresponds well with the case of regularized all-channel, less with all-channel and the least with multi-channel deconvolution. The same performance was observed when comparing the spread of GadoSpin-P AIFs. The spread of Magnevist AIFs was the lowest for multi-channel, then for regularized all-channel and the highest for all-channel deconvolution.An example anatomical image with tumor outline is shown in Fig.2 and the corresponding perfusion maps (as a direct result of spatially regularized all-channel blind deconvolution) is shown in Fig.3. The effect of spatial regularization can be observed as spatial consistency of the maps with no visible outliers and noise.
DISCUSSION AND CONCLUSION
The regularized all-channel blind deconvolution method led to the most consistent AIF estimates as it employs the available information to the largest extent. The results indicate that the proposed approach might lead to reliable blind-deconvolution AIF estimation and its wider acceptance. The method needs to be evaluated on a more complete preclinical dataset and on clinical data.Acknowledgements
MEYS, LM2018129 - National Infrastructure for Biological and Medical Imaging.References
1. Fluckiger JU, Schabel MC, DiBella EVR. Model-based blind estimation of kinetic parameters in dynamic contrast enhanced (DCE)-MRI. Magn Reson Med. 2009;62(6):1477-1486.
2. Kratochvíla J, Jiřík R, Bartoš M, Standara M, Starčuk Z, Taxt T. Distributed capillary adiabatic tissue homogeneity model in parametric multi-channel blind AIF estimation using DCE-MRI. Magn Reson Med. 2016;75(3):1355-1365.
3. Schabel MC, Fluckiger JU, DiBella EVR. A model-constrained Monte Carlo method for blind arterial input function estimation in dynamic contrast-enhanced MRI: I. Simulations. Phys Med Biol. 2010;55(16):4783-4806.
4. Bartoš M, Rajmic P, Šorel M, Mangová M, Keunen O, Jiřík R. Spatially regularized estimation of the tissue homogeneity model parameters in DCE‐MRI using proximal minimization. Magn Reson Med. 2019;82(6):2257-2272.
5. Jiřík R, Taxt T, Macíček O, et al. Blind deconvolution estimation of an arterial input function for small animal DCE-MRI. Magn Reson Imaging. 2019;62:46-56.
6. Johnson JA, Wilson TA. A model for capillary exchange. Am J Phys. 1966;210(6):1299-1303.
7. Garpebring A, Ostlund N, Karlsson M. A Novel Estimation Method for Physiological Parameters in Dynamic Contrast-Enhanced MRI: Application of a Distributed Parameter Model Using Fourier-Domain Calculations. IEEE Trans Med Imaging. 2009;28(9):1375-1383.
Figures