1575
Reproducibility of Left Ventricular CINE DENSE Strain in Pediatric Subjects with Duchenne Muscular Dystrophy1Cardiovascular Institute, Stanford University, Mountain View, CA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3University of California, Los Angeles, Los Angeles, CA, United States, 4Stanford University, Mountain View, CA, United States
Synopsis
Left ventricular (LV) peak mid-wall circumferential strain (Ecc) and twist are early biomarkers for evaluating the subtle and highly variable onset and progression of cardiomyopathy in pediatric subjects with Duchenne muscular dystrophy (DMD). Cine Displacement Encoding with Stimulated Echoes (DENSE) has proven sensitive to changes in Ecc and twist, but has not been reported in a DMD cohort. We show that free-breathing DENSE CMR at 3T provides highly reproducible middle-ventricular Ecc, radial strain (Err), and twist measurements in healthy boys and boys with DMD without evidence of late gadolinium enhancement.
BACKGROUND
Cardiomyopathy is the leading cause of mortality in boys with Duchenne muscular dystrophy (DMD). Reduced left ventricular (LV) ejection fraction (EF<55%) is a widely used marker of cardiac function and outcomes1, but the decline in LVEF is a relatively late finding among DMD patients. Late gadolinium enhancement (LGE) MRI is the gold standard for detecting focal myocardial fibrosis, but positive LGE is also a late finding in DMD2,3. We seek to define a sensitive MRI biomarker to evaluate cardiac involvement in DMD prior to the decline in LVEF or the appearance of LGE.LV peak mid-wall circumferential strain (Ecc) using tagging is an early biomarker of dysfunction in DMD4-6. In addition, peak LV twist using tagging was significantly decreased in DMD patients compared with healthy boys7. However, patients with DMD can have difficulty with the breath-holding required for tagged images. Free-breathing Cine Displacement Encoding with Stimulated Echoes (DENSE) is sensitive to changes in Ecc and twist8. Reproducibility of cine DENSE, however, has not been assessed in a pediatric cohort. Thus, our objective was to quantify the intra-user repeatability, and inter-user and intra-exam reproducibility of mid-LV Ecc, Err, and twist derived from free-breathing cine DENSE.
METHODS
LGE(-) boys with DMD (N=10, 12.5±3.0 years old) and healthy boys (N=10, 13.0±2.0 years old), were prospectively enrolled in an IRB-approved study, consented, and underwent a 3T (Skyra, Siemens) CMR exam (Table 1). Single-slice, short-axis, mid-ventricular data (Figure 1) was acquired using navigator-gated free-breathing cine DENSE (2.5x2.5x8 mm3, TE/TR=1.2/15ms, ke=0.08cycles/mm, Number of averages=3, ~2.5min/slice)9. Twit (°, degree) of regional myocardial tissue was measured as the angle between lines connecting the center of myocardial curvature and a moving myocardial pixel during the cardiac cycle. As summarized in Table 2, whole-slice peak mid-wall Ecc, radial strain (Err), and twist were computed from DENSE images using the open-source DENSE analysis tool10,11.For the intra-exam reproducibility, each of the 20 subjects underwent consecutive DENSE acquisitions (i.e., Scan-1 and Scan-2) without repositioning during a single MRI exam. Post-processing was carried out by User-1. The post-processing analyses for all 20 subjects from the first DENSE acquisition (Scan-1) were repeated by User-2 for the inter-user reproducibility. User-1 also repeated the post-processing analyses for Scan-1, with at least two weeks in between, for the intra-user repeatability. The reproducibility of whole-slice Ecc, Err, and twist were quantified using Pearson’s correlation coefficient R2, Bland-Altman analysis, the coefficient of variation (CV)12, and the intraclass correlation coefficient (ICC)13. CV was considered excellent for CV ≤ 10%, good for 10% < CV ≤ 20%, fair for 20% < CV ≤ 40%, and poor for CV > 40. ICC values were considered excellent for ICC > 0.74, good for ICC 0.6 < ICC ≤ 0.74, fair for ICC 0.4 < ICC ≤ 0.59, poor for ICC < 0.4.
RESULTS
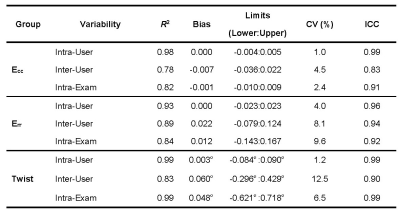
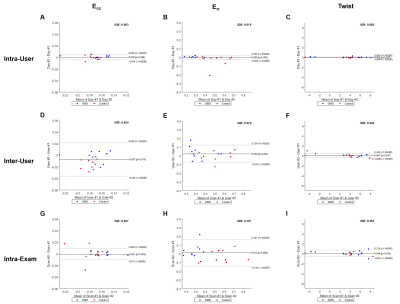
As summarized in Table 3, whole-slice Ecc provided excellent intra-user [CV=1.0%, ICC=0.99], inter-user [CV=4.5%, ICC=0.83], and intra-exam [CV=2.4%, ICC=0.91] reproducibility. Whole-slice Err also provided excellent intra-user [CV=4.0%, ICC=0.96], inter-user [CV=8.1%, ICC=0.94], and intra-exam reproducibility [CV=9.6%, ICC=0.92]. The reproducibility of twist was good-excellent for inter-user [CV=12.5%, ICC=0.90] comparison, and excellent for intra-user [CV=1.2%, ICC=0.99] and intra-exam [CV=6.5%, ICC=0.99] comparisons. The intra-user, inter-user and intra-exam measures of Ecc, Err, and twist were highly correlated (R2 ≥ 78%). For Ecc and twist, column 1 and 3 of Figure 2 demonstrate narrow limits of agreement and small biases for all comparisons. For Err, column 2 of Figure 2 demonstrate similar biases, but wider limits of agreement for all comparisons as compared to the corresponding plots for Ecc and twist.DISCUSSION
Intra-user repeatability was excellent for all measurements with all CoVs ≤ 4% and ICCs ≥ 96%, which agrees well with a previous report on healthy adults (CoV ≤ 6%, ICC = 99%14). Inter-user reproducibility was also excellent for Ecc and Err with all CoVs ≤ 9%, which agrees well with another previous report on healthy adults (CoV ≤ 8%15). Overall for all measurements, the intra-user repeatability was found to be uniformly lower than the intra-exam reproducibility. And the intra-exam reproducibility was also uniformly lower than inter-user reproducibility, which indicates that the user variability was higher than the scan variability in the study. Thus, the inter-user reproducibility is the limiting factor in the overall reproducibility of Ecc measured by DENSE. Longitudinal strain studies in LGE(-) boys with DMD are needed to better understand if changes in strain are a more sensitive indicator of cardiac involvement than LGE positivity.CONCLUSION
Free-breathing DENSE CMR at 3T was shown to provide highly reproducible whole-slice Ecc, Err, and twist measurements in the middle ventricles of healthy boys and LGE(-) boys with DMD.Acknowledgements
We are especially grateful to our co-investigator and friend, Richard Patrick Magrath III who passed away in July of 2020. NIH R01 HL131975 to DBE and NSF DGE 1650604 to NGM.References
1 Solomon, S. D. et al. Influence of Ejection Fraction on Cardiovascular Outcomes in a Broad Spectrum of Heart Failure Patients. Circulation 112, 3738-3744, doi:10.1161/circulationaha.105.561423 (2005).
2 Tandon, A. et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. Journal of the American Heart Association 4, e001338 (2015).
3 Silva, M. C. et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J. Am. Coll. Cardiol. 49, 1874-1879, doi:10.1016/j.jacc.2006.10.078 (2007).
4 Magrath, P. et al. Cardiac MRI biomarkers for Duchenne muscular dystrophy. Biomark. Med. 12, 1271-1289, doi:10.2217/bmm-2018-0125 (2018).
5 Hor, K. N. et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J. Am. Coll. Cardiol. 53, 1204-1210, doi:10.1016/j.jacc.2008.12.032 (2009).
6 Ashford, M. et al. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation 112, 2462-2467 (2005).
7 Reyhan, M. L. et al. Effect of free-breathing on left ventricular rotational mechanics in healthy subjects and patients with duchenne muscular dystrophy. Magn. Reson. Med. 77, 864-869, doi:10.1002/mrm.26137 (2017).
8 Wehner, G. J. et al. Validation of in vivo 2D displacements from spiral cine DENSE at 3T. J. Cardiovasc. Magn. Reson. 17, 5, doi:10.1186/s12968-015-0119-z (2015).
9 Zhong, X., Spottiswoode, B. S., Meyer, C. H., Kramer, C. M. & Epstein, F. H. Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magn. Reson. Med. 64, 1089-1097, doi:10.1002/mrm.22503 (2010).
10 Gilliam, A. D., Suever, J. D. & and contributors. DENSEanalysis, <Retrieved from https://github.com/denseanalysis/denseanalysis> (2016).
11 Spottiswoode, B. S. et al. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Trans. Med. Imaging 26, 15-30, doi:10.1109/tmi.2006.884215 (2007).
12 Bland, J. M. & Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. The lancet 327, 307-310 (1986).
13 McGraw, K. O. & Wong, S. P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1, 30 (1996).
14 Lin, K. et al. Reproducibility of cine displacement encoding with stimulated echoes (DENSE) in human subjects. Magn. Reson. Imaging 35, 148-153, doi:10.1016/j.mri.2016.08.009 (2017).
15 Suever, J. D. et al. Right Ventricular Strain, Torsion, and Dyssynchrony in Healthy Subjects Using 3D Spiral Cine DENSE Magnetic Resonance Imaging. IEEE Trans. Med. Imaging 36, 1076-1085, doi:10.1109/TMI.2016.2646321 (2017).
Figures

Table 1: Demographics of healthy controls and LGE(-) boys with DMD.
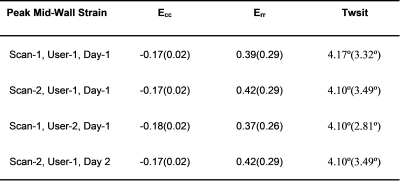
Table 2: Peak mid-wall strain results. Data is reported as median (IQR).