1569
Radiofrequency Array Concepts for Cardiac MRI at 10.5 T and 14.0 T: Does an Increased Channel Count Really Help?1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Technische Universität Berlin, Chair of Medical Engineering, Berlin, Germany, 3MRI.TOOLS GmbH, Berlin, Germany, 4Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany
Synopsis
The gain in signal-to-noise ratio is driving the ultrahigh field and extreme field MRI. This work highlights the opportunities and electromagnetic constraints of realistic radiofrequency array concepts tailored for cardiovascular MRI at 7.0T, 10.5T, and 14.0T. For this purpose electromagnetic field simulations were performed using 32, 48 and 64-channel RF transceiver array configurations at 7.0T, 10.5T, and 14.0T. Our findings demonstrate, that a higher channel count increases the degrees of freedom of B1+ shaping. Furthermore, the preliminary results provide a strong mandate for static PTX or even dynamic PTX tailored at the heart at 10.5T and 14.0T.
Introduction
The gain in signal-to-noise ratio (SNR) is a major driving force for research into ultrahigh field (UHF) MR.1,2 Progress in RF-coil design and advancement of imaging techniques facilitated a growing portfolio of clinically relevant UHF-MR applications. The success at 7.0 T MR provides convincing evidence for even higher magnet field strengths. The field has already taken further steps into the future to push the magnetic field strength boundaries into extreme field (EF) MR.3 This envisions human MR at 14.0 T and at 20.0 T4,5, and is an important conceptual leap. Unlocking the potential of EF-MR requires unravelling and leveraging the electrodynamics of the short wavelength regime. Responding to the challenges and recognizing the opportunities, this study elucidates the electrodynamic constraints at high spin excitation frequencies and explores multi-element RF transceiver array concepts tailored for human cardiovascular MR (CMR) at 10.5 T and 14.0 T. To increase the degree of freedom for transmission field shaping the channel count is increased for RF arrays of Self-Grounded Bow-Tie (SGBT) antenna building blocks. Electromagnetic field (EMF) simulations are performed at 10.5 T and at 14.0 T and benchmarked against the 7.0 T reference.6Methods
The transceiver SGBT antenna building block was scaled to the wavelength at 10.5 T, and 14.0 T using the 7.0 T configuration as a starting point.6 To investigate the impact of the channel count 32-channel and 48-channel configurations were examined at 10.5 T. At 14.0 T 32-, 48- and 64-channel configurations were designed and investigated (Figure 1). EMF simulations were performed with CST Microwave Studio (CST Studio Suite 2020, Dassault Systèmes, Vélizy-Villacoublay Cedex, France) using the human voxel model Duke.7 Post processing was performed in Matlab 2019b (MathWorks, Natick, MA) including tuning and matching, channel wise B1+ calculation, and SAR10g calculation. For B1+ shimming a genetic algorithm (GA) as well as a multi-objective GA of the Matlab global optimization toolbox was used. To benchmark the proposed array configurations shim-independent measures were calculated including: intrinsic SNR (ISNR) and intrinsic transmit efficiency (ITXE), as well as geometry-factor (g-factor) for sensitivity encoding (SENSE) parallel imaging.Results
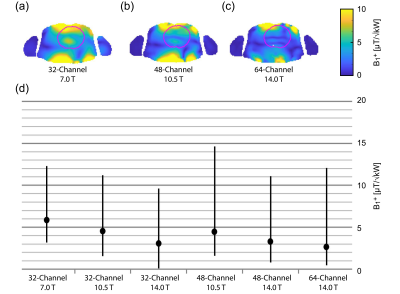
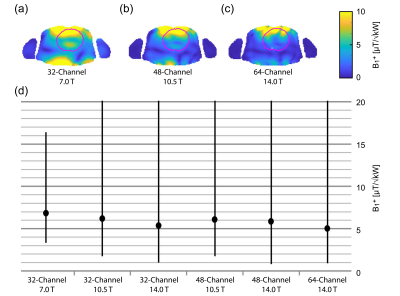
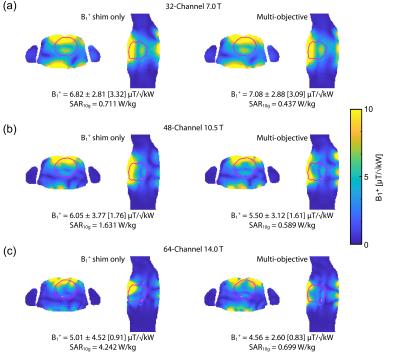
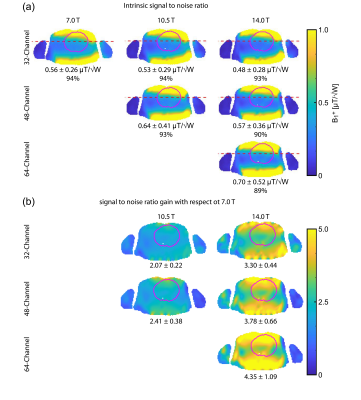
Figure 2 shows B1+ distribution deduced for GA optimization of min(B1+) using phase shimming (CTX). Figure 3 shows the same optimization but with static parallel transmit (PTX). PTX at 14.0 T yielded an increased min(B1+) for the 32-channel and the 64-channel configurations. The other configurations didn’t show a significant min(B1+) improvement, but mean(B1+) was improved for all configurations. The multi-objective optimizer helped to reduce SAR by 39% with only 7% reduction in min(B1+) for the 32-channel 7.0 T configuration (Figure 4). The 48-channel (10.5 T) and the 64-channel (14.0 T) configurations yielded a SAR reduction of 64% and 84% respectively with ~9% loss in min(B1+) (Figure 4). Figure 5a highlights the results obtained for ISNR; ITXE revealed comparable results and are not shown in this abstract. The ISNR gain combined with the signal gain of B0² in NMR relative to 7.0 T are shown in Figure 5b.8 With a constant channel count of 32-channels the mean SNR gain was found to be 2.07 for 10.5 T and 3.30 for 14.0 T for the heart. Increasing the channel count to 64 for 14.0 T resulted in an SNR gain of 4.35 ± 1.09. For the 64-channel configuration at 14.0 T the max(g-factor) = 1.72 for R=6 was shown (R-L phase encoding). This value is comparable to the 32-channel configuration at 7.0 T at R=4 with max(g-factor) of 1.67.Discussion and Conclusion
Our EMF simulations show that the compact SGBT building block permits high density and high channel count RF arrays that provide RF penetration and transmission field uniformity suitable for CMRI at 14.0 T. Beyond the channel count our findings demonstrate that increasing the degree of freedom by using static PTX instead of CTX benefits B1+ shimming. Our observations revealed that moving to EF increases SAR10g. This SAR10g increase can be offset using multi objective optimization GA for B1+ shimming. For ISNR and ITXE the theoretically achievable values could be increased at 10.5 and 14.0 T with a higher channel count facilitating a gain in SNR and TXE. The high channel count enhances the parallel imaging performance. To facilitate high density RF arrays a SGBT antenna building block was used in this work but the RF array concepts can be adapted and expanded to RF array configurations using other building blocks such as loops or hybrids of loops and dipole antenna.To conclude, a higher channel count increases the degrees of freedom of B1+ shaping tailored for CMR at extreme fields. Our preliminary results underline the benefit of more sophisticated transmission field shimming versus conventional CTX. This provides a strong mandate for static PTX or even dynamic PTX at 10.5 T and 14.0 T. These findings are heartening and provide the technical foundation for the development of RF array technology dedicated to cardiac MRI at 14.0 T.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement No 743077 (ThermalMR).References
1. Vaughan JT, Garwood M, Collins CM, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24-30. doi:10.1002/mrm.1156
2. Pfrommer A, Henning A. The ultimate intrinsic signal‐to‐noise ratio of loop‐ and dipole‐like current patterns in a realistic human head model. Magn Reson Med. 2018;80(5):2122-2138. doi:10.1002/mrm.27169
3. Niendorf T, Barth M, Kober F, Trattnig S. From ultrahigh to extreme field magnetic resonance: where physics, biology and medicine meet. Magn Reson Mater Physics, Biol Med. 2016;29(3):309-311. doi:10.1007/s10334-016-0564-1
4. Winter L, Niendorf T. Electrodynamics and radiofrequency antenna concepts for human magnetic resonance at 23.5 T (1 GHz) and beyond. Magn Reson Mater Physics, Biol Med. 2016;29(3):641-656. doi:10.1007/s10334-016-0559-y
5. Budinger TF, Bird MD, Frydman L, et al. Toward 20 T magnetic resonance for human brain studies: opportunities for discovery and neuroscience rationale. Magn Reson Mater Physics, Biol Med. 2016;29(3):617-639. doi:10.1007/s10334-016-0561-4
6. Eigentler TW, Kuehne A, Boehmert L, et al. 32‐Channel self‐grounded bow‐tie transceiver array for cardiac MR at 7.0T. Magn Reson Med. June 2021:mrm.28885. doi:10.1002/mrm.28885
7. Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55(2):N23-N38. doi:10.1088/0031-9155/55/2/N01
8. Edelstein WA, Glover GH, Hardy CJ, Redington RW. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med. 1986;3(4):604-618. doi:10.1002/mrm.1910030413
Figures