1554
Automated subject-specific pixels selection for improved image reconstruction in free-running coronary MRA using SIMBA
Ludovica Romanin1,2, Christopher W. Roy2, Milan Prsa3, Tobias Rutz4, Estelle Tenisch2, Matthias Stuber2,5, and Davide Piccini1,2
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3Division of Pediatric Cardiology, Woman-Mother-Child Department, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Service of Cardiology, Heart and Vessel Department, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3Division of Pediatric Cardiology, Woman-Mother-Child Department, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Service of Cardiology, Heart and Vessel Department, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
This work proposes an automated subject-specific individual pixels selection as an alternative to a fixed coil selection for the initialization of the input data to a similarity-driven multi-dimensional binning algorithm (SIMBA) for free-running motion-suppressed whole-heart acquisitions. By selecting timeseries with a high low-frequency energy content, we include only pixels with respiratory and cardiac information. Compared to the original method, this leads to a more accurate choice of end-expiration and diastolic phases for the reconstruction of sharp whole-heart and coronary images. Moving forward, the method needs to be refined, optimized and tested to further improve the image quality.
Introduction
SIMBA was proposed as a fast data-driven approach for the reconstruction of static motion-consistent 3D whole-heart images from untriggered and ungated free-running acquisitions1. This approach relies on the clustering of reference datapoints, i.e. superior-inferior (SI) projections, regularly acquired during the scan. The clustering is based on physical similarities in the signals, irrespective of their timepoint of acquisition. The original implementation (SIMBAc) uses the whole extent of the SI projections of the four central elements of the body array coil, located at the center of the chest, as an input to target the heart and liver2. However, in some instances SIMBAc fails at isolating precise motion states, leading to suboptimal image quality. We therefore propose a novel approach (SIMBAp) that consists of an automated subject-specific pixels selection to extract the signal components that can best promote motion consistency for SIMBA. This new algorithm was tested in a series of ferumoxytol-enhanced scans obtained from 15 patients with congenital heart disease.Methods
Pixels selection: First, all the SI projections were concatenated in time, for each coil, obtaining a 3D matrix $$$S$$$ of size ($$$N_{pixels} \text{x} N_{segments} \text{x} N_{coils}$$$). For each pixel $$$p$$$ for each coil $$$c$$$, the power spectral density $$$s(p,c)$$$ of each timeseries $$$t(p,c)$$$ is computed. $$$s(p,c)$$$ was then split into two parts, one encompassing only low-frequency components $$$s_{low}(p,c)$$$, i.e. containing relevant physiological information, and a high-frequency one $$$s_{high}(p,c)$$$, containing mainly components from trajectory imperfections. Considering only $$$s_{low}(p,c)$$$, the low-frequency energy of each pixel is calculated as $$$E(p,c) = \sum s_{low}(p,c,f)^2$$$. Finally, the pixel selection was set to include pixels with an energy value higher than an empirically selected 15% of the maximal energy at low frequencies (Figure 2). At this point, the final matrix $$$\hat{S}$$$ has a size of ($$$M \text{x} N_{segments}$$$), with $$$M$$$ being the total number of selected pixels. Finally, $$$\hat{S}$$$ was low-pass filtered to minimize the effect of non-physiological components.SIMBA: The main steps of SIMBA were implemented as in the original publication1 as illustrated in Figure 1.
Data acquisition: Fifteen congenital heart disease patients (17$$$\pm$$$10 years; 56$$$\pm$$$26 kg; 13 males) were scanned on a 1.5T (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany), after injection of ferumoxytol (2mg/kg). All datasets were acquired using a prototype free-running GRE sequence1 with a 3D radial phyllotaxis trajectory3, and the ECG recorded.
Data analysis: The low-dimensional space (Figure 1) was characterized in terms of compactness of the largest cluster, inversely related to the mean distance of each point from the centroid, the number of segments in the largest cluster, and the trajectory uniformity1. Analysis of the cardiac data selection for SIMBAc and SIMBAp was performed by calculating the percentage of diastolic data, taking the QT interval from the ECG as the duration of systole4. The respiratory data selection was assessed using a self-gating respiratory signal extraction5.
Finally, the image quality for the two algorithms was compared in terms of blood-myocardium sharpness6, the visible length and sharpness of the right coronary artery (RCA), and those of the combined left main (LM) + left anterior descending coronary artery (LAD), determined using the Soap-Bubble software tool7.
Results
Pixels selection: SIMBAp always resulted in a smaller input matrix, with an average of 83$$$\pm$$$42 pixels vs 764 obtained with SIMBAc. In only 5 of 15 cases the selected pixels were all located in the four coil elements chosen by SIMBAc.Physiological data selection: For 7 of 15 cases, SIMBAp resulted in a more accurate selection of end-expiration, and in 5 of these 7 cases in a more precise selection of diastolic data: SIMBAp 86.8$$$\pm$$$13.8% vs SIMBAc 67.7$$$\pm$$$32.5% (Figure 3A). This greatly improved the image quality (Figure 3B), resulting in a significantly higher blood-myocardium sharpness (P<0.05). Moreover, these instances were the ones for which the pixels selection differed for up to 75% from the standard body coil selection of SIMBAc.
Data analysis: The compactness of the largest cluster was significantly higher for SIMBAp compared to SIMBAc, while both trajectory uniformity and size of the largest cluster were not different (Figure 4A). A comparison of the mean blood-myocardium sharpness, visible length and sharpness of RCA and LM+LAD for all 15 patients didn’t show any statistically significant differences between the two methods (Figure 4B). Figure 5 shows an example reconstruction with both methods.
Discussion
In this work we developed and tested an alternative approach to the original fixed coil selection for the creation of the input data matrix for SIMBA. We demonstrated the advantages of an automated subject-specific selection driven by the frequency spectrum of single pixels from the SI projections of individual coil elements. This new approach improved the physiological data selection, resulting in sharper images where the original approach was incapable of adequately capturing all physiological components. Using SIMBAp we observed some instances where a good suppression of respiratory motion was not paralleled by an equally good suppression of cardiac motion. The algorithm’s steps should be optimized to improve the results in these cases as well. We plan to further improve this technique, also considering different dimensionality reduction and clustering methods. Moreover, we would like to test this pixel selection for the self-gating signal extraction as part of the 5D free-running framework.Acknowledgements
No acknowledgement found.References
- Heerfordt J, Whitehead KK, Bastiaansen JAM, et al. Similarity‐driven multi‐dimensional binning algorithm (SIMBA) for free‐running motion‐suppressed whole‐heart MRA. Magn Reson Med 2021;86:213–229.
- Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Respiratory self-navigation for whole-heart bright-blood coronary MRI: Methods for robust isolation and automatic segmentation of the blood pool. Magn Reson Med. 2011;68:571–579.
- Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magn Reson Med. 2011;66:1049-1056.
- Roes SD, Korosoglou G, Schär M, et al. Correction for heart rate variability during 3D whole heart MR coronary angiography. J Magn Reson Imaging. 2008;27:1046-1053.
- Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn Reson Med. 2019;82:2118-2132.
- Ahmad, R., Ding, Y., & Simonetti, O. P. (2015). Edge sharpness assessment by parametric modeling: application to magnetic resonance imaging. Concepts in Magnetic Resonance Part A, 44(3), 138-149.
- Etienne A, Botnar RM, Van Muiswinkel AMC, Boesiger P, Manning WJ, Stuber M. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med. 2002;48:658-666.
Figures
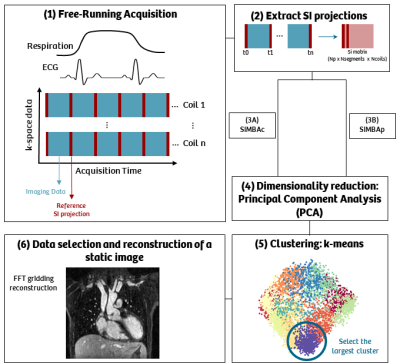
The SImilarity-driven Multi-dimensional Binning Algorithm (SIMBA).
The reference data matrix, either after coil selection (SIMBAc) or pixel selection (SIMBAp), is reduced in dimension using a dimensionality reduction method, as principal component analysis (PCA). The data, projected in this low-dimensional space, is clustered in different groups using a k-means algorithm. The largest group of data is selected, and a final 3D static image obtained by performing a gridding reconstruction.
The reference data matrix, either after coil selection (SIMBAc) or pixel selection (SIMBAp), is reduced in dimension using a dimensionality reduction method, as principal component analysis (PCA). The data, projected in this low-dimensional space, is clustered in different groups using a k-means algorithm. The largest group of data is selected, and a final 3D static image obtained by performing a gridding reconstruction.
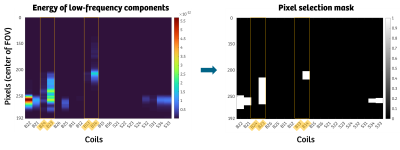
Pixel selection according to the energy of the low-frequency component of the superior-inferior (SI) projection, at the center of the field-of-view (FOV). Highlighted in yellow the selection of the four central chest coil elements that are chosen in the original implementation. In this example, we can see how a fixed body coil selection misses part of the physiological information, located in the low-frequency components of the SI.

(A) SIMBAp results in a more compact group of selected data (red) in the low-dimensional space, compared to SIMBAc (left column). This is reflected in a more accurate selection of end-expiration (central column) and diastole (right column). (B) The improved physiological data selection in SIMBAp leads to the reconstruction of significantly sharper images (blood-myocardium sharpness: 1.77 vs. 1.53). For this dataset, we also observe a significantly higher RCA sharpness for SIMBAp (43.9% vs. 42.8%).
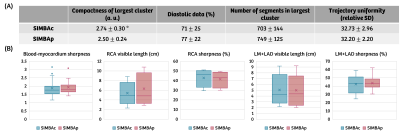
(A) SIMBAp results in a more compact cluster (*P<0.05, with a two-sided paired sample t-test). The percentage of diastolic data, number of selected segments and trajectory uniformity are not significantly different (P>0.05). (B) None of the measures (blood-myocardium sharpness, RCA visible length and sharpness, LM+LAD visible length and sharpness) are significantly different between the two methods (P>0.05). However, when the difference in the data selection between SIMBAp and SIMBAc is substantial, the sharpness measures improve for SIMBAp (Figure 3).
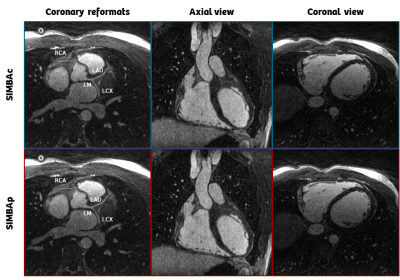
Example of a reconstructed image in the axial and coronal views, together with the reformats of the proximal tracts of all main coronaries. Both cases result in a sharp diastolic image of the heart, and clearly visible coronary arteries. The SIMBAc image is slightly more undersampled (13.7% data), than the SIMBAp one (14.2%).
DOI: https://doi.org/10.58530/2022/1554