1546
Striatal tau deposition in mild cognitive impairment revealed by removal of iron-related off-target binding effects in 18F-AV1451 PET1Center for Advanced Neuroimaging, University of California Riverside, Riverside, CA, United States, 2Department of Bioengineering, University of California Riverside, Riverside, CA, United States, 3Department of Psychology, University of California Riverside, Riverside, CA, United States
Synopsis
We use tissue susceptibility to control for iron-related off-target binding effects in 18F-AV1451 PET and examine the impact of APOE-ε4 carrier status on striatal tau-PET signal in mild cognitive impairment. We found significant increases in tau-PET SUVR in the putamen (p=0.01) and caudate nucleus (p=0.046) of APOE-ε4 positive participants compared to APOE-ε4 negative participants. Controlling for striatal iron, significant correlations were seen between striatal tau-PET SUVR memory measures in the control (putamen: r=0.435; caudate: r=0.623) and APOE-ε4 positive MCI (putamen: r=0.403; caudate: r=0.648) groups with greater tau burden correlated with greater memory impairment.
Introduction
Patients with mild cognitive impairment (MCI) experience declines in cognition that do not meet the threshold for dementia, but are likely to convert to Alzheimer’s disease (AD)1. AD and MCI pathology include the accumulation of b-amyloid into extracellular plaques and hyper-phosphorylated tau into intracellular neurofibrillary tangles (NFTs)2,3. Individuals carrying the apolipoprotein E-ε4 (APOE-ε4) allele are at increased risk of developing AD pathology4.Tau deposition is assessed using the radioligand 18F-AV1451, which is known to bind with neuromelanin and iron in addition to tau NFTs5,6. This off-target binding obstructs measurement of tau burden in iron-rich deep gray matter structures, like the striatum. Postmortem AD studies have reported the presence of NFTs in the striatum7,8,9. However, in vivo examination of striatal NFTs in MCI and the impact of APOE-ε4 carrier status remain largely unexplored due to off-target binding effects of the 18F-AV1451 radioligand. We use quantitative susceptibility mapping (QSM) to quantify iron and remove iron-related off-target binding effects in the striatum and examine striatal tau burden in APOE-ε4 positive (i.e. one or more APOE-ε4 alleles) MCI, APOE-ε4 negative MCI, and APOE-ε4 negative control participants.
Methods
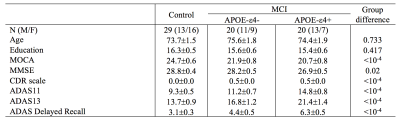
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) was queried for individuals diagnosed with MCI who had APOE-ε4 data, 18F-AV1451 PET (denoted tau-PET) and multi-echo gradient echo MRI images acquired at the same visit. MCI diagnoses were based on a subjective memory concern reported by a clinician, abnormal memory function on the education-adjusted Logical Memory II subscale, and a clinical dementia rating greater than 0.5. A total of 20 APOE-ε4 negative (ε4-) MCI, 20 APOE-ε4 positive (ε4+) MCI, and 29 control subjects met these criteria. Participants with either one or two APOE-ε4 alleles were considered APOE-ε4 (+). Demographic information is shown Table 1.All MRI data were acquired on Siemens Prisma scanners. Anatomic images were acquired with a T1-weighted MP-RAGE sequence (echo time (TE)/repetition time (TR)/inversion time=2.98/2300/900 ms, flip angle=9°, voxel size=1.0×1.0×1.0 mm3) and were used for registration to common space and correction of partial volume effects in the PET data.
Multi-echo data were collected with a 2D gradient recalled echo sequence (TE1/∆TE/TR = 6/7/650 ms, voxel size=0.86×0.86×4 mm3, 44 slices). Quantitative susceptibility maps (QSM) images were estimated in MATLAB using custom scripts.
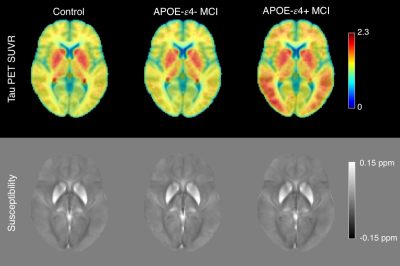
18F-AV1451 PET (tau-PET) imaging was performed at each ADNI site according to standardized protocols. The 18F-AV-1451 protocol entailed injection of 10 mCi of tracer followed by an uptake phase of 80 min during which the subjects remained out of the scanner. 18F-AV-1451 emission data were collected as 4×5min frames. PET imaging data were analyzed with FSL and PET partial volume correction (PETPVC) toolbox. 18F-AV1451 PET scans were motion corrected, averaged, registered to the participant's own T1-weighted MRI image. Grey matter, white matter, and CSF maps were segmented in the T1-weighted image and used to correct for partial volume effects. The median standardized uptake value (SUV) in the inferior cerebellar cortex was chosen as a reference and used to calculate mean SUV ratio (SUVR) in cortical ROIs. Figure 1 shows mean SUVR and susceptibility maps.
The Harvard-Oxford atlas was used to create striatal regions of interest (ROIs; caudate, putamen), we then measured mean susceptibility and tau-PET (SUVR) metrics in each ROI.
Results
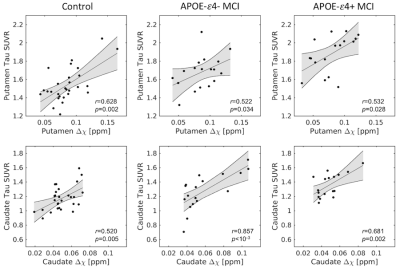
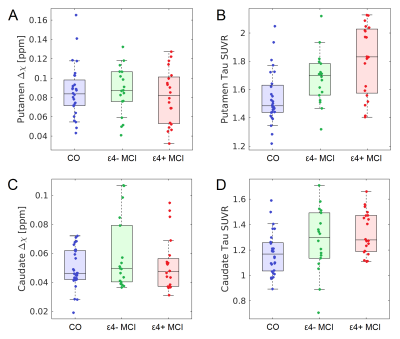
Significant correlations (Figure 2) between striatal tau-PET measures and susceptibility were observed for each group in caudate (control: r=0.520,p=0.005; ε4-: r=0.857,p<10-3; ε4+: r=0.681,p=0.002) and putamen (control: r=0.628,p=0.002; ε4-: r=0.522, p=0.034; ε4+: r=0.532,p=0.028).The effect of group (APOE-ε4+ MCI, APOE-ε4- MCI, control) was tested with separate analysis of covariance (ANCOVA) in each striatal ROI, controlling for susceptibility (see Figure 3). For putamen, there was a significant main effect in group (p<10-4; F=14.962), with pairwise-comparisons of the marginal means showing higher putamen tau-PET SUVR in APOE-ε4+ MCI relative to APOE-ε4- MCI (p=0.008) and control (p<10-3) groups. Similarly, for caudate nucleus, there was a significant main effect in group (p<10-4; F=29.811), with marginal means comparisons showing higher caudate nucleus tau-PET SUVR in APOE-e4+ MCI relative to APOE-ε4- MCI (p=0.046) and control (p=0.008) groups.
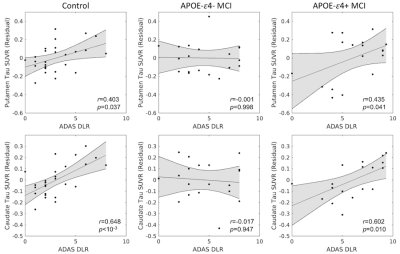
The relationship between tau-PET SUVR and delayed recall was assessed with Pearson correlations controlling for susceptibility, separately for each group. Significant correlations were seen between delayed recall and striatal tau-PET SUVR in the APOE-ε4+ (putamen: r=0.435; caudate: r=0.623) and control (putamen: r=0.403; caudate: r=0.648) groups with greater tau burden correlated with more forgotten words. No significant correlations were seen between striatal tau-PET SUVR and delayed recall in the APOE-ε4- (putamen: r=0.017; caudate: r=-0.001) group.
Discussion
We found evidence of increased tau pathology (tau-PET SUVR) in the striatum of MCI participants relative to controls, with APOE-ε4+ MCI exhibiting the highest tau burden. This work agrees with histology studies showing higher tau NFTs in the striatum7,8,9. Interestingly, higher tau pathology in the striatum was related to worse memory performance (more words forgotten in ADAS delayed recall) in APOE-ε4 positive MCI and control participants. These results accord with earlier studies showing verbal recall is linked to striatal integrity10. Taken together, these findings suggest that APOE-e4 allele increases the risk of developing AD pathology in the striatum.Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.References
[1] Albert, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:270–279.
[2] Morris, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67:122-131
[3] Schmechel, et al. Increase amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 90:9649-0653
[4] Roses and Saunders. APOE is a major susceptibility gene for Alzheimer's disease. Curr Opin Biotechnol. 5:663-667.
[5] J. Y. Choi et al., Off-Target (18)F-AV-1451 Binding in the Basal Ganglia Correlates with Age-Related Iron Accumulation. J Nucl Med 59:117-120.
[6] Spotorno, et al. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain. 143:1341-1349
[7] Selden, et al. Human striatum: the distribution of neurofibrillary tangles in Alzheimer’s disease. Brain Research. 648:327–331
[8] Braak and Braak. Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J. Neuropathol. Exp. Neurol. 49:215-224
[9] Braak and Braak. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82:239–259.
[10] Landau, et al. Striatal Dopamine and Working Memory. Cereb Cortex. 19:445–454
Figures